Chapter 4
advertisement

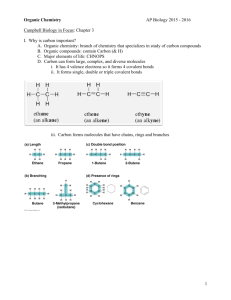
Objectives Carbon and Molecular Diversity of Life • Explain how carbon’s electron configuration accounts for its ability to form large, complex, and diverse organic molecules. • Describe how carbon skeletons may vary, and explain how this variation contributes to the diversity and complexity of organic molecules. • Describe the basic structure of a hydrocarbon and explain why these molecules are hydrophobic. • Distinguish among the three types of isomers: structural, geometric, and enantiomer. • Name the major functional groups found in organic molecules. Describe ● ● Organic chemistry is the study of carbon compounds Organic compounds range from simple molecules to colossal ones • The concept of vitalism is the idea that organic compounds arise only within living organisms ● ● It was disproved when Friedrich Wohler accidently synthesized urea from ammonium cyanate in1828 Vitalism crumbled completely after several decades of laboratory synthesis of increasingly complex organic compounds The formation of bonds with carbon ● ● ● ● ● The key to an atom's chemical characteristics is its electron configuration The electron configuration of carbon gives it covalent compatibility with many different elements Carbon has 6 electrons, with 2 in the first electron shell and 4 in the second shell; thus, it has 4 valence electrons in a shell that holds 8 electrons Carbon can form four covalent bonds with other carbon atoms - linked carbon atoms form the backbone of organic molecules Molecular Diversity arising from carbon skeleton variation ● Carbon chains form the skeletons of most organic molecules – chains vary in length and shape • The carbon backbone may be straight, branched or arranged in closed rings ● ● Some carbon skeletons have double bonds, which vary in number and location Such variation in carbon skeletons is one important source of the molecular complexity and diversity that characterize living matter ● ● The versatility of carbon’s bond forming is the basis for isomers Isomers molecules with the same molecular formula but different structures and properties – three types of isomers • structural-differ in the covalent arrangements of their atoms • cis-trans- carbons have covalent bonds to the same atoms, but these atoms differ in their spatial arrangements due to the inflexibility of double bonds • enantiomers- mirror images of each other and that differ in shape due to the presence of an asymmetric carbon Hydrocarbons ● Hydrocarbons are molecules consisting of only carbon and hydrogen – hydrocarbons are found in many of a cell’s organic molecules ● ● Atoms of hydrogen are attached to the carbon skeleton wherever electrons are available for covalent bonding Although hydrocarbons are not prevalent in most living organisms, many of a cell's organic molecules have regions consisting of only carbon and hydrogen The Pharmacological importance of enantiomers ● Important to pharmaceutical industry as molecules used as drugs often have enantiomers which have no or undesired effects • thalidomide – one enantiomer acts as sedative, other causes severe birth defects • L-dopamine and D-dopamine – one useful in treating Parkinson’s disease, other has no effect ● ● Methamphetamine also occurs in two enantiomers that have very different effects One enantiomer is highly addictive stimulant, other has much weaker effect (found in OTC for nasal congestion Functional Groups ● ● Functional groups covalently linked to the carbon backbone of hydrocarbons give the molecule distinctive chemical properties Functional groups important in the chemistry of life: – hydroxyl (-OH): polar, dissolves in water – carbonyl (=CO): polar, dissolves in water – carboxyl (-COOH): hydrogen dissociates; weak acid – amino (-NH2): accepts hydrogen; basic – sulfhydryl (-SH): can form disulfide bonds – phosphate (-PO4): acidic and polar, dissolves in water – methyl (-CH3): non-polar • Addition of different side groups to the same backbone significantly changes a molecule’s action ● ● ● Consider the differences estradiol (female sex hormone) with testosterone (male sex hormone) Both are steroids, organic molecules with a common carbon skeleton in the form of four fused rings These sex hormones differ only in the chemical groups attached to the rings • Living matter consists mainly of carbon, oxygen, hydrogen, and nitrogen, with smaller amounts of sulfur and phosphorus ● ● The versatility of carbon makes possible the great diversity of organic molecules At the foundation of all biological diversity lies this variation at the molecular level