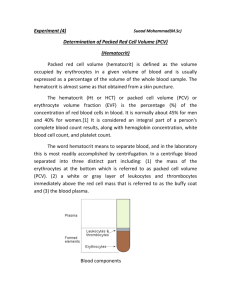
Noninvasive monitoring of blood composition
advertisement

PSI-SR-1252
Noninvasive monitoring of blood composition
Nicusor V. Iftimia
Daniel X. Hammer
Dave I. Rosen
Chad E. Bigelow
R. Daniel Ferguson
Nicusor V. Iftimia, Daniel X. Hammer, Dave I. Rosen, Chad E. Bigelow, R. Daniel
Ferguson, "Noninvasive monitoring of blood composition ," presented at SPIE Photonics
West (San Jose, CA), (21-26 January2006).
Copyright © 2006 Society of Photo-Optical Instrumentation Engineers.
This paper was published in SPIE Photonics West, and is made available as an electronic
reprint (preprint) with permission of SPIE. One print or electronic copy may be made for
personal use only. Systematic or multiple reproduction, distribution to multiple locations
via electronic or other means, duplication of any material in this paper for a fee or for
commercial purposes, or modification of the content of the paper are prohibited.
Downloaded from the Physical Sciences Inc. Library. Abstract available at
http://www.psicorp.com/publications/sr-1252.shtml
Noninvasive monitoring of blood composition
Nicusor V. Iftimia*, Daniel X. Hammer, Dave I. Rosen, Chad E. Bigelow,
and R. Daniel Ferguson
Physical Sciences Inc., 20 New England Business Center, Andover, MA 01810
ABSTRACT
A novel instrument for real-time in vivo measurement of blood composition is presented. Two optical technologies are
combined in this instrument: spectral domain low coherence interferometry (SD-LCI) and retinal tracking. Retinal
tracking is used to stabilize the LCI beam on vessels. SD-LCI is used to get depth-reflectivity profiles within the
vessels. Multiple signals are rapidly acquired, averaged and processed. Differences in the slopes of the depth reflectivity
profiles for different subjects correspond to the difference in the scattering coefficient, which is proportional to the
concentration of red blood cells per cubic mm of blood (hematocrit). Preliminary measurements on several healthy
volunteers show a good correlation with the normal range of the hematocrit.
Keywords: blood hematocrit, low coherence interferometry, retinal imaging.
1. INTRODUCTION
In this paper we show noninvasive measurement of blood composition (e.g. hematocrit, hemoglobin, etc.) by using
spectral domain low coherence interferometry (SD-LCI) and directly accessing small vessels of the retina.
Blood hematocrit is defined as the proportion, by volume, of the blood that consists of red blood cells (RBC’s). Noninvasive monitoring of hematocrit is of importance in several clinical applications. Periodic or continuous monitoring of
hematocrit is valuable in clinical environment for patients with blood disorders, as well as in emergency situations.
Several noninvasive methods have been proposed for hematocrit measurements. Ultrasound based continuous
hematocrit measurement has been proposed by Johner et al.1. They determined the value of hematocrit by monitoring
changes in ultrasound wave velocity propagation in plasma as a function of RBC’s concentration. Although they
reported very good agreement, they noted that the uncertainty of the method depended markedly on even minute
temperature variations. A Doppler ultrasound method has been tested as well by Secomski et al.2 Their results
demonstrated that hemotocrit could be non-invasively determined in the brachial artery to within an error of 5%.
However, it has been observed that the lateral movement of the vessel induces additional errors. To keep the error
within 5% very sophisticated multiple gated Doppler circuitry is necessary. Near infrared (NIR) optical imaging of the
blood vessels through skin has been tested by Schmitt et al.3 for noninvasive hematocrit measurement. Unfortunately,
lack of direct access to blood vessels alters the result of the measurements due to variation in skin optical properties
from one person to another.
SD-LCI is largely used in ophthalmology to obtain cross-sectional or en face OCT images of the retina4-6, to measure
Doppler flow in the retinal vessels7,8, or to do spectroscopic measurements9,10. However, in this case LCI measurements
require long exposure durations to collect enough photons and achieve reasonable signal-to-noise levels within the
confines of laser safety requirements. Unfortunately, the movement of the eye makes these measurements even more
difficult. The light beam interrogating the eye must be kept on the same location to target a retinal vessel and collect
depth-reflectivity profiles of the blood. This is possible only by employing an eye motion stabilization technique.
*
iftimia@psicorp.com, phone 978-689-0003; fax: 978-689-3232
We demonstrate that the combination of two technologies within the same instrument, tracking line scanning laser
ophthalmoscopy (TLSLO) and SD-LCI, can be successfully used to stabilize eye motion and measure the hematocrit
level.
2. APPROACH AND INSTRUMENTATION
Our approach is to non-invasively measure hematocrit by employing low coherence interferometry (LCI). As shown in
Figure 1, LCI uses a broadband light source and an interferometer to provide high-resolution fringe data. When the
reference arm of the interferometer is scanned, a high-resolution reflectivity profile of the sample is obtained (see
Figure 2).
Figure 1. Schematic of the LCI system.
Figure 2. Typical LCI depth reflectivity
profile.
A relatively simple theoretical model can be used to extract hematocrit information from LCI measurements. The slope
of the axial reflectivity profile is a function of the scattering coefficient, which means that by simply measuring its slope
the concentration of red blood cells can be deducted.
The LCI signal is proportional to the square root of the light intensities Is and Ir returning from the sample and the
reference arm of the interferometer, respectively:
S LCI ( z ) = k[ I r I s (λ , z )]1/ 2
(1)
k denotes a constant factor, which is given by the LCI system specifications (e.g. detector responsitivity). Is(λ,z)
depends on the reflectivity of the sample at a depth z within the sample, the signal intensity caused by coherent
superposition of the electromagnetic fields from different scatters within the coherence volume, and the position of the
focal spot within the sample.
The intensity of the light from the sample arm that propagates through the sample can be described by Beer’s law:
I s ( λ , z ) = I s 0 ( λ ) exp{ −[ µ a ( λ , z ) + µ s ( λ , z )] z } ,
(2)
where Is0 denotes the incident intensity, λ the wavelength, µa the absorption coefficient and µs the scattering
coefficient.
It is to be noted that for the LCI case mostly single scattered photons are detected, which means that the total scattering
coefficient is more appropriate to be used in our calculation instead of the reduced scattering one. If we consider the
total scattering coefficient, take the logarithm of Eq. (1), and use Eq. (2) we obtain:
ln[ S LCI ( z )] = ln{k [ I r ( λ ) I s 0 ( λ )]1/ 2 } − {[ µ a ( λ , z ) + µ s ( λ , z )] z} ,
where we have taken into account the double pass configuration of LCI.
(3)
With the assumption of a constant incident and reference intensity, Eq. (3) is a linear function in z and the slope s is
given by:
(4)
s = −[ µ a (λ , z ) + µ s (λ , z )]
Measurements performed by other research groups11,12 show that at around 800 nm µa is almost two orders of
magnitude lower than µs (µa ≅ 0.82 mm-1, µs ≅ 57 mm-1), which suggests that the slope of the signal is mainly
dependent on the scattering coefficient. This means that slope changes in Eq. (4) are a direct measure of the scattering
coefficient, which is proportional to hematocrit.
Based on the above arguments, the hematocrit level can be estimated directly by measuring the slope of the LCI depth
reflectivity profile and applying a scaling factor that can be determined by taking measurements in blood samples with a
known hematocrit value.
In our measurements an advanced system using the spectral domain LCI approach was used. This approach uses an
alternative method of retrieving depth information. It examines the cross-spectral density to reconstruct the
interferogram by detecting the interference signal as a function of wavelength13,14. This method does not require
modulation of the reference arm length and therefore has potential for faster acquisition rates. Recent work has
experimentally demonstrated a 148-fold (21.7 dB) sensitivity improvement with SD-LCI13.
However, because of the relatively high absorption of the blood, LCI measurements require long exposure durations to
collect enough photons and achieve reasonable signal-to-noise levels within the confines of laser safety requirements.
Unfortunately, eye movement makes these measurements even more difficult. The light beam interrogating the eye must
be kept on the same location to target a retinal vessel and collect depth-reflectivity profiles of the blood. This is possible
only by employing an eye motion stabilization technique. We have used our mature tracking line scanning laser
ophthalmoscopy (TLSLO) technique and SD-LCI to stabilize the eye motion and non-invasively measure the hematocrit
level.
The schematic of our TLSLO/SD-LCI instrument developed by us and used for hematocrit measurements is shown in
Figure 3. A photograph of the TLSLO/SD-LCI instrument is shown in Figure 4.
The TLSLO system consists of a custom-designed confocal line scanning laser ophthalmoscope (LSLO) and a retinal
tracker. A complete description of these systems can be found elsewhere15-17. A pair of tracking mirrors is used to steer
the LSLO image and the LCI beam with the movement of the eye.
Figure 3. Schematic of the TSLO/SD-LCI System.
Figure 4. Photograph of the TLSLO/SD-LCI instrument.
The SD-LCI system consists of a broadband light source, a fiber-optic interferometer, and a spectral detection and data
processing unit. The fiber optic interferometer consists of a 50/50 fiber optic beam-splitter that has four arms: the
illumination arm, the sample arm, the reference arm, and the detection arm. Light from the light source is split in two
within the sample and reference arms. A fraction of the light transmitted to the sample arm is backscattered from the
sample, passes back into the interferometer and mixes with the reference beam. This light passes back to the input arms,
being equally split between the detector arm and the illumination arm. An isolator is placed in the illumination arm to
prevent this light from going back to the light source. An optical delay line (ODL), consisting of a mirror placed on a
translation stage and a neutral density filter (NDF) that adjusts this arm’s power level, is placed in the reference arm to
adjust the length of this arm to match the length of the sample arm. The polarization of the reference beam is adjusted
with a paddle polarization controller to match the polarization of the light from the sample arm. This adjustment ensures
that polarization changes caused by bending and rotation of the optical fiber in both the sample and reference arms do
not wash out the interference fringes. The LCI beam is sent to the eye by means of a scanning mirror pair that allows
for beam positioning on the desired retinal vessel.
The TLSLO part of the system is mounted in a stand alone enclosure that can be positioned such that a patient can
comfortably look through the objective to a fixation target. The fiber optic interferometer and the spectrometer are
mounted on a separate optical breadboard. They are connected with the TLSLO and the electronics box through several
fiber optic patchcords and electrical wires. The TSLO/SD-LCI system is controlled by a P4/3 GHz computer using
LabView software. An interactive graphic user interface (GUI) is used for setting system’s parameters, data acquisition,
processing, and storage.
3. MEASUREMENTS, RESULTS, AND DISCUSSION
Ex vivo measurements on sheep blood samples (Hemostat Laboratories) and noninvasive retinal measurements on
several volunteers were performed with our instrument.
The goal of the ex vivo study was to calibrate the system and evaluate its capability to discriminate between different
hematocrit levels. Various hematocrit levels were obtained by mixing whole blood with blood serum, which was
obtained through centrifugation. The hematocrit of each blood sample was determined by measuring the ratio between
the sedimented volume of red blood cells obtained after centrifugation and the entire volume of the blood. The slope of
the LCI signal was computed and the hematocrit level was determined using a scaling factor determined from the value
of hematocrit measured via centrifugation. This scaling factor was further used for the in vivo study.
The in vivo study was performed on 4 healthy volunteers (2 males and 2 females) with ages between 37 and 60. The
measurements were performed by accessing superficial retinal vessels and analyzing averaged one-dimensional SD-LCI
axial reflectivity profiles. Eye motion stabilization was done with the TSLO instrument. Retinal SLO images were used
to localize vessels and place the LCI beam on the vessel. Multiple LCI reflectivity profiles were taken in major retinal
vessels with diameters of about 200 microns, situated within the vicinity of the nerve optic head. Six sets of multiple
LCI measurements at various time intervals were taken for each volunteer and about 10,000 LCI depth reflectivity
profiles were averaged in each data set. A comparison of the LCI reflectivity profile “in” and “out” of the vessel was
performed as well. Figures 5.b) and 5.d) show that the LCI reflectivity profile outside of the vessel reflects the structure
of the retina while the profile acquired inside the vessel shows a linear decay (logarithmic plot) specific to
backscattering and absorption of light on blood. The slope of the reflectivity profile was extracted for each set of
measurements. We have noticed that this relatively high variation of the slope is due in parts to z motion of the patient.
As a result of the z motion, the peak of the reflectivity profile moves within the A-scan. Since the sensitivity of the
spectrometer is not the same over the length of the A-scan (decreases towards the end of the A-scan because higher
modulation of the spectrum is not resolved by the limited resolution of the spectrometer), the slope of the reflectivity
profile varies and as a result the averaged value over many A-scans is affected by Z motion of the patient. However,
this artifact can be diminished by correcting the modulation transfer function (MTF) of the spectrometer and/or with z
tracking. These improvements will be incorporated in future work.
The hematocrit was computed from the slope of the LCI reflectivity profile using a scaling factor determined from the
sheep blood measurements. However, since ex vivo clinical measurements on human blood were not included in our
IRB protocol, this scaling factor might be slightly different for human blood and as a result our measured hematocrit
values are only orientative.
Figure 5. a) and c). TSLO retinal images of the same volunteer. b). Averaged LCI reflectivity profile in the circled point in a).
d). Averaged LCI reflectivity profile in the circled point in c).
The hematocrit value for each individual was compared with the normal clinical values for both men and women. As
shown in Figure 6 our measurements yield hematocrit values that fall within the normal limits for both males and
females.
60
Male
normal range
Hematocrit [%]
50
Female
normal range
40
30
20
10
M
M
F
F
1
2
3
4
0
Volunteer
H-6579
Figure 6. Histogram of the averaged hematocrit for 4 volunteers. Six sets of measurements were performed for each volunteer. The
histogram shows a good correlation of the measured hematocrit with the normal clinical range.
4. CONCLUSIONS
Noninvasive hematocrit measurement using SD-LCI and retinal tracking has been demonstrated. Preliminary
measurements on four healthy volunteers indicate hematocrit levels within the normal range for both males and females.
Further refinement of the method and described instrumentation might allow development of a clinical tool that could
be useful for daily monitoring of blood hematocrit for patients with blood disorders. The hardware and signal
processing scheme can be easily adapted to measure other blood parameters such as oxygenation and hemoglobin.
ACKNOWLEDGEMENT
This work was supported by DARPA- USN-N66001-04-8038.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
8.
C. Johner, P. Chamney, D. Schneditz, and M. Krämer, “Evaluation of an ultrasonic blood volume monitor”,
Nephrol. Dial. Transplant. 13, 2098–2103 (1998).
W. Secomsky, A. Nowicki, F. Guidi, P. Tortoli, and P.A. Lewin, “Non-invasive measurement of blood hematocrit
in artery”, Bulletin of the Polish Academy of Sciences, 53 (3), 245-50 (2005).
J.M. Schmitt, Z Guan-Xiong, and J. Miller, “Measurement of blood hematocrit by dual-wavelength near-IR
photoplethsymography, “ Proc. SPIE 1441, 150-161 (1992).
J.F. de Boer, B. Cense, B.H. Park, et al., "Improved signal-to-noise ratio in spectral-domain compared with
timedomain optical coherence tomography," Opt. Lett. 28, 2067-2069 (2003).
N.A. Nassif, B. Cense, B.H. Park, et al., "In vivo high-resolution video-rate spectral-domain optical coherence
tomography of the human retina and optic nerve," Opt. Express 12, 367-376 (2004).
A. Gh. Podoleanu, M. Seeger, G. M. Dobre, D. J. Webb, D. A. Jackson and F. Fitzke, “Transversal and longitudinal
images from the retina of the living eye using low coherence reflectometry,” J. Biomed. Opt. 3, 12 (1998).
H. Liang, M. G. Cid, R. G. Cucu, G. M. Dobre, A. G. Podoleanu, J. Pedro, and D. Saunders, "En-face optical
coherence tomography – a novel application of non-invasive imaging to art conservation," Opt. Express 13, 61336144 (2005).
B.R. White, M.C. Pierce, N. Nassif, et al., "In vivo dynamic human retinal blood flow imaging using ultrahighspeed spectral domain optical Doppler tomography," Opt. Express 11, 3490-3497 (2004).
9.
10.
11.
12.
13.
14.
15.
16.
17.
J. Zhang and Z. Chen, "In vivo blood flow imaging by a swept laser source based Fourier domain optical Doppler
tomography," Opt. Express 13, 7449-7457 (2005).
C. Xu, D. L. Marks, M. N. Do, and S. A. Boppart, "Separation of absorption and scattering profiles in
spectroscopic optical coherence tomography using a least-squares algorithm," Opt. Express 12, 4790-4803 (2004).
M Yu Kirillin, A V Priezzhev, V V Tuchin, R K Wang, and R Myllylä, “Effect of red blood cell aggregation and
sedimentation on optical coherence tomography signals from blood samples”, J. Phys. D: Appl. Phys. 38 25822589 (2005).
André Roggan, Moritz Friebel, Klaus Dörschel, Andreas Hahn, and Gerhard Müller, “Optical Properties of
Circulating Human Blood in the Wavelength Range 400–2500 nm”, Journal of Biomedical Optics 4(1), 36-46
(1999).
A. F. Fercher, W. Drexler, C. K. Hitzenberger and T. Lasser, “Optical coherence tomography- principles and
application,” Rep. Prog. Phys. 66, 239-303 (2003).
R. Leitgeb, C. K. Hitzenberger, and A. F. Fercher, “Performance of Fourier domain vs. time domain optical
coherence tomography,” Opt. Express 11, 889-894 (2003).
S. H. Yun, G. J. Tearney, J. F. de Boer, N. Iftimia, and B. E. Bouma, “High-speed optical frequency-domain
imaging,” Opt. Express 11, 2953-2963 (2003). B. Hermann, K. Bizheva, A. Unterhuber, B. Povazay, H. Sattmann,
L. Schmetterer, A. F. Fercher and W. Drexler, "Precision of extracting absorption profiles from weakly scattering
media with spectroscopic time-domain optical coherence tomography," Opt. Express 12, 1677-1688 (2004).
D. X. Hammer, R. D. Ferguson, N. V. Iftimia, T. Ustun, G. Wollstein, H. Ishikawa, M. L. Gabriele, W. D.
Dilworth, L. Kagemann, and J. S. Schuman, "Advanced scanning methods with tracking optical coherence
tomography," Opt. Express 13, 7937-7947 (2005).
D. X. Hammer, R. D. Ferguson, J. C. Magill, M. A. White, A. E. Elsner, and R. H. Webb, "Image stabilization
for scanning laser ophthalmoscopy," Opt. Express 10, 1542-1549 (2002).