Enthalpy answers
advertisement
gChemistry
Name
2.3,1 Enthalpy Changes Exam Questions
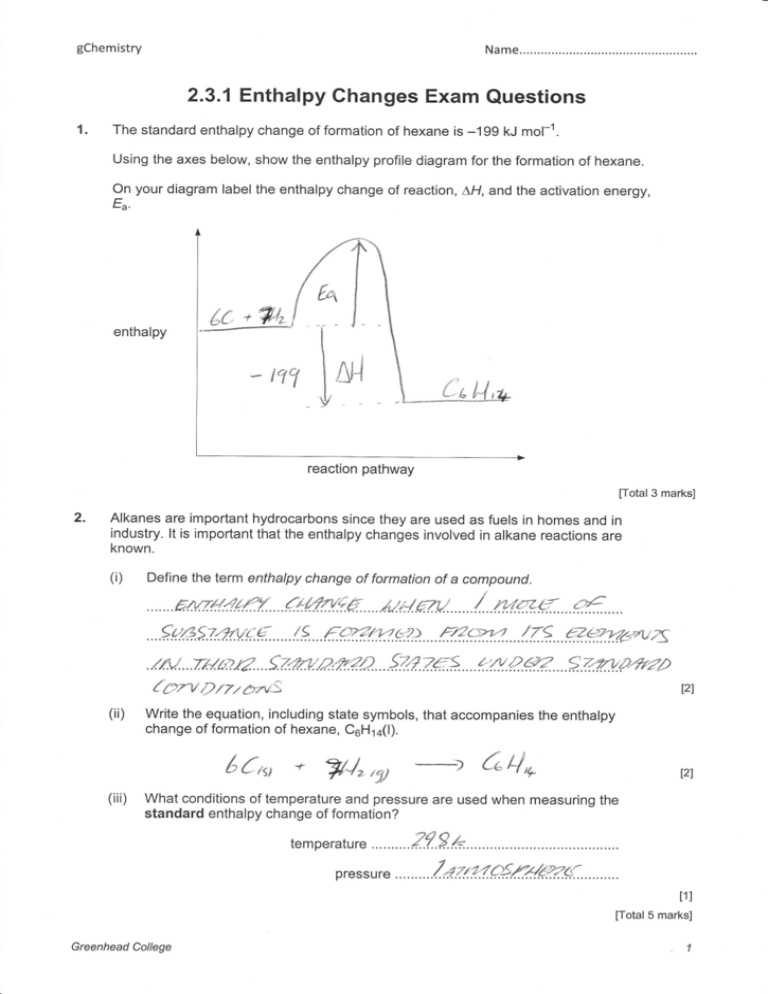
1.
The standard enthalpy change of formation of hexane is -199 kJ mol-1.
Using the axes below, show the enthalpy profile diagram for the formation of hexane.
On your diagram label the enthalpy change of reaction, LH, and the activation energy,
Ea
enthalpy
reaction pathway
[otal3
2.
marks]
Alkanes are important hydrocarbons since they are used as fuels in homes and in
industry. lt is important that the enthalpy changes involved in alkane reactions are
known.
(i)
Define the term enthalpy change of formation of a compound.
/,/Hilt/- / nzzzt+-.. €.....
{.*?Ken) FE c>14 lf.5 ez.9???su X,
aar.A.+ff . Qfft.v!€
...
.5tt*
5.2.
Arue. €. . . . . #.
.
...
...
./A/-..7/r'e.8...s.f,miap.,:ru,2- ...9A72--5...
QrzY i)t7t OntS
(ii)
.
(:(..2#-. 97"vt/'P!!'%O
I2j
Write the equation, including state symbols, that accompanies the enthalpy
change of formation of hexane, C6H14(l).
6C,ri r
(iii)
..
pJ,ry
64,u
t2l
What conditions of temperature and pressure are used when measuring the
standard enthalpy change of formation?
temperatur
"
..........
pressure
2f..9.
*
/471ezPg,a/P{
t1l
f-otal5 marksl
Greenhead College
gChemistry
3.
Name..........
The combustion of butane is shown in the equation below.
c+Hro(g)
(i)
* 6 + oz(g) -+ 4co2(g) + 5H2o(t)
2
The standard enthalpy change of combustion of butane is -2877 kJ mol-1. What
does sfandard mean in this context?
7f,91,
,
/ ezmb%./ed_
t1l
(ii)
Define the term enthalpy change of combustion.
/ mezg_ dsaSszt^/?r7
knzpAU€rc5 (e4vpz+v{ mz&t-#Z/m: /N- &
4
-z-
tv: r//Rz ..s'7(#.p.ezD 5:7,€7€s utut2c# sz."fuD&22
(ontpzT/M/J
t2l
(iii) Complete the enthalpy profile diagram for the combustion
of butane. Label the
activation energy, Eu, and the enthalpy change, AH.
enthalpy
C+Hro(g) + 6102(9)
/+tOt +;4O
progress of reaction
t3I
fl-otal6 marks]
4.
ln an experiment to determine the standard enthalpy change of combustion of propan-
Greenhead College
gChemistry
1-ol, C3H7OH, a student used the apparatus shown below.
propan-1-ol
(a)
Define the term enthalpy change of combustion.
ffiz
.dS Cr)u Z
/tt)
l2l
(b)
write the equation for the standard enthalpy change of combustion of
propan-1-ol, C3H7OH.
&ilnr;1/r,) t Scz
(c)
Tz
3Co
.* t
&l/,
o
pl
The student measured 50.0 cm3 of water into the beaker and lit the burner. When
the temperature of the water had gone up by 12.8 'c, he found that 0.100 g of
propan-1-ol had been burnt.
(i)
Calculate the energy, in kJ, produced by burning 0.100 g of propan-1-ol.
The specific heat capacity of water is 4.1 8 J g-' K t.
q)=r%f
teytO
(ii)
= Q' I
6c
KJ
/ooa
t2l
Calculate the number of moles of propan-1-ol in 0.100 g.
,rL'/(%9
(iii)
:-e,)< L,tg , l7,g energy = ..2.:.6.8.
-)
-./.--
number of moles =
o oo l6Y
l2l
Calculate the enthalpy change of combustion, in kJ mol-1, of propan-1-ol.
26V
i) 'oef Mv
-,--e
t
entharpy.n"nsJ...'n"
ot
^o,'
tl I
(d)
The student then calculated the error of the 50cm3 measuring cylinder used. The
Greenhead College
gChemistry
Name..........
maximum error of the measuring cylinder was +- 1cm3. Calculate the % error in
this piece of apparatus.
c/o
(e)
'= ? cy
*/L)o
etolcn = +
A /o
Sr)
t11
The student looked in a text book and found that the actual value for the standard
enthalpy change of combustion of propan-1-ol was more exothermic than the
experimental value.
Suggest two reasons for the difference between this value and the one he
obtained experimentally.
1
.........
.Pffi7
....
/:e2 .2.
..
.T€'.
....
7//q I u,r tzo'tz:ru,p2 NS
(*" zta7s7\
J
2 $@71am/ H
t21
(f)
The student told one of her friends and she suggested using a 10cm3 measuring
cylinder 5 times as its maximum error was +- 0.5cm3
State and explain whether this method would reduce the error in measuring
50cmo of water.
/uu&,7/Pt€
us€t h trrwes
p7/%fl
o5
(-7
t) -=
.:'___-=-
(g)
/o
l1l
x S x/OC:
uJ/(-L'
B€ lylutTtPZll?D 5 rtr"t b
= 25%
€zzaszpz rl/e/ltt
U)
The student repeated the experiment using the same apparatus. The water
started at 60oc. The student was surprised to find the value was less
exothermic. Explain why.
11I
/S e7 ,1 /-/t€t/t?z 7t<z'w@87u/?6
-fil€ -9etzEawx,//>/L$
. ' . i1'I),ry€ //gzg7 /,-,// / t3€ /csr Ta
Tate /"J47gz
t rt?.
"Z
y /d//1 /J"lr,/€
fi=,zz€
erf 7tr'F h/.47H
(h) Other students in the
got
Et/4?Or€zl7cb
545v744{
class
very similar enthalpy changes of combustion even
though their temperature changes and masses of propan - 1 - ol burnt were
different. Explain this in terms of energy and
moles.
t1l
[Total 15 marks]
7r lffiz rasfft?z4v&ti€$ rl/,ffi/*'e{ /S
FPAlfuYvf UPF',' 7//€ /-,'J lF iaft)Zp,S tf
Pe57e'6rY * / - t>z
9ratteW
Greenhead College
ti u ;€rv/
0Mt
/u"r/L
B{
gChemistry
5.
Name
A student carries out an experiment to determine the enthalpy change of combustion of
glucose.
tt ll,u Ot
,z
glucose
ln the experiment, 0.831 g of
is burned. The energy released is used to heat
100 cm3 of water from 23.7 'C to 41 .0 'C.
(i)
Calculate the energy released, in kJ, during combustion of 0.831 g glucose.
The specific heat capacity of water = 418 J g-' K '.
Density of water = 1.00 g cm-3.
Q-
Y:4:4T
iocc)
/oox/'/9*lY3
6
a. tz
energy = .....T....(.J......
f<.t
t2t
(ii)
Calculate the amount, in moles, of glucose that is burned.
flle69-= o
Y^3i
/3o
amount
=
O oc*'62
t2t
(iii) Calculate
the enthalpy change of combustion of glucose.
Give your answer to three significant figures.
4/J- Y23 : -is6b? + -is7
o aoQ;6'Z
AH6
=
... kJ mol-1
l2l
[otal6
6.
marks]
Some reactions of H2O2 are exothermic. Use ideas about the enthalpy changes that
take place during bond breaking and bond making to explain why some reactions are
exothermic.
rw(w6 a/e.U€.F_.- /: zez(ffiW /sr/:4../1/t#u'
-&was E&. m ...Z//'.ffi/''ru{€.P-u&?{ y E&}st64D
lf otal2
Z) f&g6#/4 'ft/€ //u/v/aL- {-*wDS
Greenhead College
marksl
gChemistry
7.
The equations for the combination of gaseous atoms of carbon and hydrogen to form
methane, CHa, ?nd ethane, CzHo, are shown below.
C(g) + 4H(g) -+
CHa(g)
LH = -1652 kJ mot-1
2c(g) + 0H(g) -+ c2H6(g) LH = -2825 kJ mol-1
Use this data to calculate:
(i) the bond enthalpy of a C-H bond,
bond enthalpy =
i6 s2
(ii)
l
Eezaer% "8a'
Lttlt 8€
F-U,.kV/&Ur(
45 &ntPS4Pd&Qw.cn:
.........
u k)YA L/4LL//-'S
kJ mol-1
ht S t t,ruu/''
-- 4
7t crts
t1l
the bond enthalpy of a C-C bond.
bond enthalpy =
.........
kJ mol-1
.\f-q z zszs f '-c = 78zs * 2t+19
= M1'
zAYg + (c*c) =zszeJ
(A-r-r,f)
[otal
3 marks]
Enthalpy changes can be calculated using enthalpy changes of combustion. The table
below shows some values for standard enthalpy changes of combustion.
(azalErts'udrt'l . . fitfllfcv'E
LHcelkJ mol-1
^
DCe4Al
Use these values to calculate the sta;rdard enthalpy change of the reaction below.
@@
C(s) + 2Hz@) -+ CHa(s)
LIJ- *146
sqo)
- ?aa
CO, '* LU, A
standard enthalpy
+ Ylo
change
_
.--,r
4 9Qo
/
I ?......
kJ mot-1
[Total3 marks]
Greenhead College
gChemistry
Enthalpy changes of combustion can be used to determine enthalpy changes of
formation.
(i)
Write the equation for the standard enthalpy change of formation of butane,
C+Hro. lnclude state symbols in your answer.
ACtil ! SA*A- ep,or,)
l2l
(ii)
Use the following data to calculate the standard enthalpy change of formation of
butane.
standard enthalpy chanse
.f(ffi@fkJ
carbon
-394
hydrogen
-286
butane
-2877
prr\ o..
C
kCTq -t S9tllr'9) -->
ht-3qA=-rs;,\"J, -2gA'-l4=.\-* tuoA
I.to*
Greenhead College
-+ 2
.F
-. 'f e zsYY)
+7gYv
-,t
s[/"-D
answer
- zao|
mot-1
"' , fWecz.s
po^t tt/
v7+ =
kJ mol-1
/2? /-J/4d-!otarSmarksl
gChemistry
10.
The standard enthalpy change of combustion of glucose can also be determined
indirectly.
Calculate the standard enthalpy change of combustion of glucose using the standard
enthalpy changes of formation below.
substance
6{wmor-1
C6H12O6(s)
-1250
coz(g)
-394
H2O(r)
-286
e) 60z(g)--
CeHrzOa(s) +
Evw/#/ciu
'^ Vv2vaanf
uP"
aD
6Oz(q)-- 6COz(9)
6COr(q) + 6Hzo(l)
6H2O(l
\,
$
'
(tzsa)\..
\ \-
.A/ 6^ - 4?4' = - !36+
g,-ze6=-tilb
.,
I
-.
t tzgo
- +-o8b
6cst r gtl t eo,
+/
^so
+' 40ga
answer =
....*..2.9.'fu
. kJ mor-1
fl-otal3 marks]
Greenhead College








