Mini SDS-PAGE Gel Protocol for the Bio-Rad Mini
advertisement

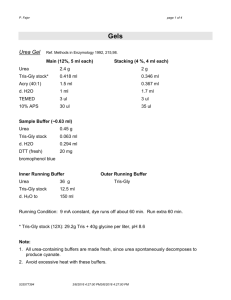
Mini SDS-PAGE Gel Protocol for the Bio-Rad Mini-Protean Tetra Cell Note: For best results, use MilliQ or nanopure equivalent water to prepare all buffers and samples. Preparing 1X SDS PAGE Running Buffer 10X Tris Glycine SDS PAGE Running Buffer Components Tris Glycine SDS Nanopure water Amount for 1 L 30.3 g 144.1 g 10 g Adjust to 1 L Dilute to 1X for use. The 1X can be made several days in advance. You will need 800 ml buffer for 1-2 gels and 1200 ml for 3-4 gels. Note: If the 10X or 1X buffer is turning yellow, the glycine is degrading and so it is time to properly dispose of it. Sample Preparation 1) Prepare the samples: 10-Well Gels- maximum loading volume 30 µl/well (1.0 mm thick) 15-Well Gels- maximum loading volume 15 µl/well (1.0 mm thick) Sample Water Laemmli Sample Buffer (2X) Β-mercaptoethanol Total Volume 10 μl 2.0 μl 10 μl 2) Prepare 1X sample buffer to fill the empty wells: Water Laemmli Sample Buffer (2X) Β-mercaptoethanol Total Volume 7.5 μl 1.5 μl 15 μl 10 μl 2.0 μl 20 μl 12.5 μl 2.5 μl 25 μl 15 μl 3 μl 30μl 400 μl 500 μl 100 μl 1000 μl 3) Incubate sample(s) at 95°C for 5 min. Note for people who are working with membrane proteins: Do not boil your sample because it may cause your proteins to aggregate. Instead, load your sample immediately after adding the loading buffer. 1 Preparing a Precast Gel for Electrophoresis 1) 2) 3) 4) Remove the precast gel from the storage pouch. Rinse the outside of the gel thoroughly with distilled water. In one fluid motion, gently remove the comb. Rinse the wells thoroughly with either: distilled water or 1X running buffer. Invert the gel and shake to remove excess buffer/water. Repeat twice. If necessary, straighten the sides of the wells. 5) Remove the tape from the bottom of the Precast Gel cassette to expose the bottom edge of the gel. 6) Repeat for any remaining gels. Assembly of the Mini-Protean Tetra Cell 1) On a clean flat surface, open the clamping frame on the Electrode Assembly (see Figure 4a below). When running only 1-2 gels, use the Electrode Assembly (the one with the banana plugs), NOT the Companion Running Module (the one without the banana plugs). 2) Place the first gel cassette (with the short plate facing inward) onto the gel supports molded into the bottom of the clamping frame assembly surface (see Figure 4a). Note that the gel will now rest at a 30° angle, tilting away from the center of the clamping frame (see Figure 4b). Note: Please use caution when placing the first gel. Make sure that the clamping frame remains balanced and does not tip over. 3) Place the second gel on the other side of the clamping frame, again by resting the gel onto the supports (see Figure 4b). If running an odd number of gels (1 or 3), use the buffer dam. At this point there will be two gels resting at an angle, one on either side of the clamping frame, tilting away from the center of the frame. 4) Using one hand, gently pull both gels towards each other, making sure that they rest firmly and squarely against the green gaskets that are built into the clamping frame. 5) While gently squeezing the gel cassettes against the green gaskets with one hand (keeping constant pressure and both gels firmly held in place), slide the green arms of the clamping frame over the gels, locking them into place. Alternatively, you may choose to pick-up the entire assembly with both hands. While making sure that the gels do not shift, simultaneously slide both arms of the clamping frame into place (see Figure 4c). 6) Check to make certain that the short plates sit just below the notch at the top of the green gasket. 2 7) Place the Electrode Assembly on the bench top. Fill the inner chamber on the Electrode Assembly with 1X SDS PAGE Running Buffer (Figure 4d). The buffer should be just under the top edge of the outer gel plate (200 ml). Check to see that the buffer is not leaking from the inner chamber onto the bench top. Gel Supports Put buffer here Sample Loading 1) Using a gel loading pipette tip, load the molecular weight standards (usually in lane 1). 2) Load your samples into the appropriate wells by lowering the pipette tip to the bottom of the sample well and slowly pipet the sample into well. Be careful not to contaminate another well with the sample or puncture the bottom of the well. 3) Load 20 µl 1X Sample loading buffer into any remaining empty wells. This promotes uniform running of the stacking front. Running Gel(s) 1) Place the tank with the front of the tank facing you (the front of the tank is the face that has the 2-Gels and 4-Gels line markings). When oriented properly, the red marking on the top inside edge of the tank will be on your right, and the black marking on the top inside edge of the tank will be on your left. 2) Place the Electrode Assembly (banana plugs) so that this assembly is in the back position of the cell, making sure that the red (+) electrode jack matches the red marking on the top right inside edge of the tank. 3 3) If running 3-4 gels, place the the Companion Running Module (no banana plugs) in the front position. Make sure again that red (+) electrode is matching with the red marking on the top inside right edge of the tank. CAUTION: When running only 1-2 gels, DO NOT place the Companion Running Module in the tank. Doing so will cause excessive heat generation and prevent electrophoretic separation. 4) Fill the tank (lower chamber) with 1X SDS PAGE Running Buffer to the indicated level (550 ml for 2 gels and 680 ml for 4 gels). 5) Place the Lid on the Mini-PROTEAN Tetra Tank. Make sure to align the color coded banana plugs and jacks. The correct orientation is made by matching the jacks on the lid with the banana plugs on the electrode assembly. 6) Insert the electrical leads into a suitable power supply with the proper polarity. For 1-2 gel: PowerPac Basic or PowerPac 200 For 3-4 gels: use any PowerPac Basic or PowerPac HC 7) Apply power to the Mini-PROTEAN Tetra Cell and begin electrophoresis: Standard Method 200 V until the dye front reaches the bottom (expected run time 31-39 minutes) Low Voltage Method-Membrane Proteins and Bands for Mass Spectrometry Analysis 40 V for 10 min (allows better stacking of the proteins) 100 V until the dye front reaches the bottom (expected run time 80-90 minutes) Note: The low voltage method reduces the amount of heat which is generated during electrophoresis resulting in sharper bands. For further cooling, the gel maybe ran in a coldroom/coldbox. Don’t prechill the running buffer since colder temperatures will cause the SDS to come out of solution. Gel Removal 1) At the completion of the run, turn off the power supply and disconnect the electrical leads. 2) Remove the tank lid and carefully lift out the electrode assemblies. Pour off and discard the running buffer into the proper hazardous waste container. Note: Always pour off the buffer before opening the arms of the assembly to avoid spilling the buffer. 3) Open the arms of the assembly and remove the gel cassettes. 4) Open the cassette by aligning the arrow on the opening key with the arrows marked on the cassette. Insert the key between the cassette plates at all 4 locations and apply downward pressure to break each seal. Do not twist the lever. Gently pull apart the two plates beginning from the top of the cassette. 5) Hold the cassette plate over a container with water or transfer buffer with the gel side facing towards the container. Place the gel into the fluid and agitate gently until the gel separates from the plate. You may need to use Gel Knife to carefully loosen the lower corner of the gel and allow the gel to peel away from the plate. 6) Rinse the Mini-PROTEAN Tetra cell electrode assembly, clamping frame, and mini tank with distilled, deionized water. 4 References The protocol above was based in part, on the following literature from Bio-Rad: • Mini-PROTEAN Precast Gels: Instruction Manual and Application Guide (1658100 Rev A) • Mini-PROTEAN TGX and TGX Stain-Free Precast Gels: Quick Start Guide (Bulletin 5933 US/EG RevB) • Mini-PROTEAN TGX Precast Gels (Bulletin 5871) Supplies Below lists the supplies needed. Comparable supplies may be purchased through other vendors; however, the gels need to specifically fit the Bio-Rad Mini-Protean Tetra Cell. Gels Mini-Protean TGX Precast Gels, Any kD, 10 well, 30 µl Mini-Protean TGX Precast Gels, Any kD, 10 well, 30 µl Mini-Protean TGX Precast Gels, Any kD, 10 well, 50 µl Samples Buffer Reagents Laemmli Sample Buffer 2-Mercaptoethanol Protein Standards Precision Plus Protein Unstained Standards (Sypro Ruby) Precision Plus Protein Dual Color Standards (Western) BenchMark Unstained Protein Ladder (Sypro Ruby) BenchMark Prestained Protein Ladder (Western) Stains Sypro Ruby Fluorescent Protein Stain (10 ng sensitivityovernight) Gelcode Blue Stain Reagent (25 ng sensitivity-overnight) Size Vendor Catalog Number 2 gels/box 10 gels/box 10 gels/box Bio-Rad Bio-Rad Bio-Rad 456-9033S 456-9033 456-9034 30 ml 25 ml Bio-Rad Bio-Rad 161-0737 161-0710 1 ml 500 µl 2 x 250 µl 2 x 250 µl Bio-Rad Bio-Rad Life Technologies Life Technologies 161-0363 161-0374 10747012 10748010 200 ml Life Technologies S12001 500 ml Thermo Scientific Pierce 24590 Homemade Coomassie Blue (100 ng sensitivity) Imperial Protein Stain (>10 ng sensitivity) 200 ml Thermo Scientific Pierce Bio-Rad 161-0495 2.5 L 4L EMD/VWR EMD/VWR EMD-AX0073-9 EM-MX0475-1 1L Oriole Fluorescent Gel Stain Fixer/Destain Solution Acetic Acid Methanol Written by Melissa Sondej (version 04/17/12) 5 24615