Chapter 22, Organic Chemistry
advertisement

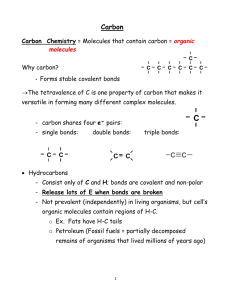
Aside on Chapter 22, Organic Chemistry Why is organic chemistry important: 1) Materials 2) Energy (oil & coal) 3) Human health a) diagnosis b) treatment (drugs) 4) A drug development logic progression identify lesion (malfunction) or key target site (pathogen) 9 Determine structure of site (X-ray?) 9 Build (synthesize) molecule to bind site 9 Test for efficacy (repeat steps as needed) 1 Drug Examples: 1) estrogen dependent breast cancer raxolifene and tamoxifen see PDBSum entry: 2qxs http://www.ebi.ac.uk/pdbsum/2qxs 2 2) Cystic fibrosis (l-o-f): Kalydeco Approved for patients w/ G551D mutation 3) Metastatic melanoma: Approved for patients w/ V600E BRAF mutation 3 4) HIV protease inhibitor saquinavir See: http://www.ebi.ac.uk/pdbsum/2nmz 4 Chapter 22, Organic Chemistry I. The Nature of Organic Molecules A. C is tetravalent. Implications? B. C forms covalent bonds. Could you have predicted? 1. Non-polar 2. Polar C. C forms single, double, & triple bonds. (Orbital hybridization? See pp. 166-171) 1. sp3 for bonds 2. sp2 for & bonds 3. sp for & bonds & bonds or 4. Structural/functional implications? See Dr. Winter’s Orbitron: 5 http://winter.group.shef.ac.uk/orbitron/ Why/how do covalent bonds form? Stability issues (How do p+ and e! relate to this?) Charge issues (+ and !) Orbital issues 6 Orbital Hybridization: Hybrid names are clues to their origin? sp3 CH4 NH3 H2O Energy level diagram to show how sp3 orbitals can be viewed as arising from atomic orbitals. Do energy levels & structure. 7 8 Next overlap orbitals from separate C and H atoms to form covalent bonds. sp3 hybridization often accurately describes single bonding arrangements for Period 2 atoms C, N, O, & F. 9 10 What about Period 2 atoms with multiple bonds? Try an atom with one double bond first. Draw energy level diagram: Bonds occupying the space directly between nuclei are sigma (ó) bonds. This is the prime real estate. The “other” bond of double (or triple) bonds has two lobes, is called a pi (ð) bond, and it occupies the space above and below the sigma bond. It forms when the non-hybridized p lobes of the 2 p shell overlap to form a bond. The outcome for ethylene (C2H4) looks like this: 11 12 We aren’t going to get into sp hybridization much this semester, but you will intensively in organic. However, with what types of bonding patterns would you encounter sp hybridization? & I. Alkanes (& isomerism) A. Hydrocarbons (H & C only) 1. Linear (straight-chain) We’ll get to naming shortly. Only one way to assemble each of these. 2. Branched-chain Some times many different ways that structures can be branched. B. Isomers: same molecular formula, different structure. Try probs. 22.1-2 p. 903. 13 14 III. Drawing Organic Structures A. Standard, but be careful in Flatland. B. Condensed. You must know element valences to use this correctly. Prob. 22.3, p. 904, Key Concept Prob. 22.4 C. Line structures. 1. End of each line is , unless other symbol pres. 2. Fill carbon valence to 4 with . Do Prob. 22.3 with line structures. IV. The Shapes of Organic Molecules A. This means getting out of Flatland A perspective representation: 15 B. Rotation can occur around most “true” single bonds. (More on “true” later, but basically, some things that we draw as single bonds really aren’t.) Look at conformations of ethane. 16 V. Naming alkanes (structure based) A. Learn the IUPAC system: prefix-parent-suffix prefix-parent-family B. Parent name: based on the longest C chain, Table 22.1 C. Suffix is based on the dominant functional group D. Prefix: essentially everything else. Probs 22.7-8 b, c, p. 909. Key Concept Prob. 22.9, p. 909. VI. Cycloalkanes A. Naming 17 B. For cyclohexanes (& bigger) conformers (boat/chair) C. Geometric isomers (cis- and trans-) (limited rotation) Prob. 22.10,a & b, 22.11 c, p. 911. 18 VII. Reactions of alkanes (organization of organic texts?) A. Oxidation (requires extreme conditions) 1. With excess O2(g): C8H18 + O2 ÷ + 2. With limiting O2(g): CO is produced instead of CO2! C8H18 + O2 ÷ B. Halogenation CO + uv C2H6 + Cl2 ÷ + HCl C. Not a whole lot else, which is helpful for you. Think of hydrocarbons as relatively non-reactive. Reactivity increasing as you introduce functional groups. 19 VIII. Organic Functional Groups A. Multiple bonds between C atoms (alkenes & alkynes) Check orbital hybridization again. B. Bonds between C atoms and non-C atoms (heteroatoms). See Table 22.2, p. 913, try Probs. 22.13 & 14, p. 914. Commit functional group names/structures to memory. IX. Alkenes and Alkynes (unsaturated hydrocarbons) A. Alkenes 1. Structure 2. cis-/trans- isomers 3. naming 4. Relatively free rotation occurs around single bonds. Why not around double bonds? (See next page.) 20 5. First step of the human visual process is a cis-trans isomerization. B. Alkynes 1. Structure 2. Naming Probs. 22.15 a & 16 c, p. 916 X. Reactions of Alkenes and Alkynes A. Addition reactions (“across double bond”) are most important. B. What gets added? (H2, hydrohalo acids, halogens, H2O) Prob. 22.17, p. 917 21 XI. Aromatic Compounds and Their Reactions A. Define aromaticity: For now, 6-membered rings with 3 alternating double bonds. Archetype is benzene. B. Resonance, a major factor in stability of organic compounds. Prob. 22.20, p. 919 22 C. Rxns.: Mostly substitutions, nitration, halogenation, sulfonation. 23 XII. Alcohols, Ethers, and Amines A. Alcohols (!OH group) 1. Naming (try some examples) 2. Synthesis by hydration of alkenes B. Ethers (!O!group) C. Amines (!NH2 group) Basicity Prob. 22.23 XIII. Aldehydes and Ketones A. Basic functionality is the carbonyl group: 1. Aldehydes: R1, R2, or both are H 2. Ketones: both R1 and R2 are C atoms 24 B. Why separate aldehydes and ketones into 2 classes? Simply, aldehydes are more readily oxidized. XIV. Carboxylic Acids, Esters, and Amides A. Carboxylic Acids: carbonyl + hydroxyl = carboxyl 1. Functional group: 2. Are these guys acidic or basic? Treatment for ant stings? B. Esters (note: the C atom would have 4 bonds) C. Amides Examples: ????? 25 Probs. 22.24 & 22.25, pp. 928. XIV. Synthetic Polymers 26