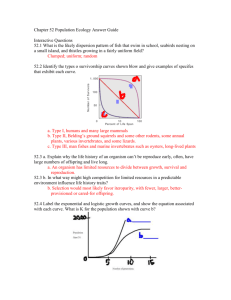
Life History
advertisement

Life History Charles W. Fox, Frank J. Messina Introduction The life history of an organism is its pattern of survival and reproduction, along with the traits that directly affect survival and the timing or amount of reproduction. Rates of survival and reproduction can be estimated across age classes, or across different stages in organisms with complex life cycles. Life history traits include growth rate; age and size at sexual maturity; the temporal pattern or schedule of reproduction; the number, size, and sex ratio of offspring; the distribution of intrinsic or extrinsic mortality rates (e.g., patterns of senescence); and patterns of dormancy and dispersal. These traits contribute directly to age-specific survival [l(x)] and reproductive [m(x)] functions in a conventional life table constructed for demographic purposes. Compared to purely morphological, behavioral, or physiological traits, life history traits have an especially strong effect on overall fitness. They can also vary widely between closely related species or even among populations of the same species. A major goal of life history theory is to determine how natural selection gives rise to this variation. Both mathematical modeling and empirical evidence can be marshaled to explain, for example, why some organisms live very briefly while others live for decades or centuries, and why some produce just a few large offspring while others produce many thousands of small offspring. General Overviews Among the general treatments of life history evolution, Stearns 1992 and Roff 1992 provide an excellent starting point for exploring the basic theory and evidence. Roff 2002 revisits many of the topics covered in the author’s previous book (Roff 1992), but with a more evolutionary-genetic framework. Charnov 1993 mainly considers dimensionless ratios of life history variables that appear to be invariant across taxa (for a criticism of this perspective, see Nee, et al. 2005). More recent reviews, including the edited volume Flatt and Heyland 2011 and the book chapter Sibly 2012, have focused on physiological mechanisms underlying animal life history traits, and their associated tradeoffs. Marshall, et al. 2012 reviews life history variation of marine invertebrates and Crawley 1997 provides an overview of plant life history ecology. Charnov, E. L. 1993. Life history invariants: Some explorations of symmetry in evolutionary ecology. Oxford: Oxford Univ. Press. Reviews much of the author’s previous work, and compares key life history characters across species to establish scaling laws and invariant, dimensionless ratios of trait pairs. This “symmetry” approach has been applied to explain variation in such features as sex ratios, aging, and determinate vs. indeterminate growth. Crawley, M. J. 1997. Life history and environment. In Plant Ecology. 2d ed. Edited by M. J. Crawley, 73–131. Oxford and Malden, MA: Blackwell Science. Overview of life history variation in plants. Available online (2009) by subscription. Flatt, T., and A. Heyland. 2011. Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Oxford: Oxford Univ. Press. An edited volume that presents summaries for a large number of topics in life history evolution, with particular attention to genetic and physiological mechanisms underlying traits and trade-offs. Marshall, D. J., P. K. Krug, E. K. Kupriyanova, M. Byrne, and R. E. Emlet. 2012. The biogeography of marine invertebrate life histories. Annual Review of Ecology, Evolution and Systematics 43:97–114. Compiles life history and geographic data for more than one thousand species of marine invertebrates, and many traits covary with temperature and local productivity. An excellent example of the utility of the comparative approach, applied here to group whose traits seem less constrained by phylogeny than is true for terrestrial species. Available online for purchase or by subscription. Nee, S., N. Colegrave, S. A. West, and A. Grafen. 2005. The illusion of invariant quantities in life histories. Science 309.5738: 1236–1239. Argues that some dimensionless ratios of key life history appear to be “invariant” only because of a lack of independence between the variables compared (e.g., offspring-weaning mass vs. maternal mass). The authors show that, for bounded variables, even random simulations of trait values can give the impression of invariance. Available online for purchase or by subscription. Roff, D. A. 1992. The Evolution of life histories: Theory and analysis. New York: Chapman & Hall. A thorough compendium of the theory and evidence used to understand life history evolution. This tour-de-force covers both genetic and optimization approaches for virtually all important traits, and undoubtedly influenced much subsequent work in this field. Roff, D. A. 2002. Life history evolution. Sunderland, MA: Sinauer. Updates Roff 1992 and devotes more attention to quantitative-genetic modeling. This book is also organized in an interesting way; instead a sequential coverage of the usual life history traits, Roff considers the evolution of relevant traits in three types of environments: constant, stochastic, and predictable. Sibly, R. M. 2012. Life history. In Metabolic ecology: A scaling approach. Edited by R. M. Sibly, J. H. Brown, and A. Kodric-Brown, 57–66. Chichester, UK; and Hoboken, NJ: Wiley-Blackwell. Metabolic rate, which varies with body size and temperature, influences rates of resource allocation to growth, maintenance, and reproduction. Sibly shows that many life history traits covary with mass, so that the metabolic theory of ecology can help identify broad patterns and constraints. Stearns, S. C. 1992. The evolution of life histories. New York: Oxford Univ. Press. An accessible and relatively concise introduction to the theory that is the foundation of life history studies. Provides a useful introductory discussion of the meanings of adaptation and fitness, and their application to the study of life histories. History The tools of life history analysis trace their origins to the beginning of demography, the study of the “vital statistics” of a population. The first demographic studies consisted of actuarial tables for human populations. Demographic analyses have been conducted for centuries, and age-specific patterns of mortality remain the foundation of the life insurance industry. The origins of ecological life history analysis are more recent, tracing back to the seminal works of David Lack in the 1940s and Lamont Cole in the 1950s. Lack was a population biologist whose main contribution to life history theory was to integrate Darwinian thinking into studies of avian life histories. He promoted quantitative testing of adaptationist hypotheses by focusing on which values of a given life history trait (such as clutch size in birds) maximize fitness. The approaches pioneered in Lack 1947 were crucial to the development of life history theory, but they contained little mathematical analysis. Cole 1954 importantly introduced mathematical thinking to the study of life histories in the context of population ecology. In his classic paper, Cole examined how changes in demographic attributes of a population affect the rate of population increase (r). Among his many observations, he raised a simple mathematical conundrum, known as Cole’s paradox (see below), which spurred a generation of ecologists to integrate mathematics into studies of life history evolution. Stearns 1976 reviews the historical developments that led to the major theoretical developments in life history ecology.Harper 1967 extended the Darwinian perspective to plant life histories, emphasized the importance of phenotypic plasticity, and considered life history variables not usually addressed by animal ecologists, such as vegetative growth. Ten years later, John L. Harper’s magnum opus, Population Biology of Plants (Harper 1977), provided a foundation for much subsequent work in plant population biology, including plant life histories. Because of these seminal papers and books, the life history literature expanded rapidly in the 1980s. Cole, L. C. 1954. The population consequences of life history phenomena. Quarterly Review of Biology 29.2: 103–137. In a groundbreaking, quantitative approach to life history evolution, Cole emphasized both the demographic consequences of variation in life history traits (for example, age at first reproduction), and the role of natural selection in producing such variation. Available online for purchase or by subscription. Harper, J. L. 1967. A Darwinian approach to plant ecology. Journal of Ecology 55.2: 247–270. With abundant quantitative examples, Harper’s Presidential Address to the British Ecological Society argued for a need to inject more quantitative analysis and evolutionary thinking into plant population ecology. Available online for purchase or by subscription. Harper, J. L. 1977. Population biology of plants. New York: Academic Press. Helped define the science of plant population biology. With respect to life histories, this book emphasized concepts that are especially relevant to plants, such as the distinction between genetic and physiological individuals, and the more complex patterns of aging and reproductive cycles. Lack, D. 1947. The significance of clutch-size. Ibis 89.2: 302–352. Provided the first important discussion of the evolution of clutch size, which had previously been explained by untenable ideas about population self-regulation. Lack explained clutch size in altricial birds as a function of selection acting at the level of the individual. Available online for purchase or by subscription. Stearns, S. C. 1976. Life-history tactics: A review of the ideas. Quarterly Review of Biology 51.1: 3–47. An early review of the major theoretical developments in life history theory, and the historical steps that gave rise to them. Availableonline for purchase or by subscription. Trade-Offs A key concept needed to solve problems like those raised by Lack and Cole is the recognition of trade-offs. Tradeoffs are linkages between traits such that a beneficial change in one results in a detrimental change in another. Without trade-offs, natural selection would favor immortal organisms that produce an infinite number of offspring. Two kinds of trade-offs have played a particularly major role in the development of life history theory: trade-offs that arise because organisms must allocate a finite amount of resources to distinct traits, and those that arise because of genetic linkages between separate life history traits. ALLOCATION TRADE-OFFS Since much of life history theory considers how resources are allocated to various components of the life cycle, allocation trade-offs—the inability of an organism to allocate resources simultaneously to different aspects of the life history—are central to the development of life history theory. Such trade-offs are reviewed in Stearns 1989 and Stearns 1992 (cited under General Overviews). Resources such as energy, time, and essential nutrients are limited and so must be divided among growth, maintenance (survival), and reproduction. Geber 1990 notes that, in plants, meristems must be allocated to growth or reproduction, creating a developmentally based trade-off. This partitioning of resources is often referred to as the principle of allocation, or Y-model of allocation, and leads to tradeoffs between all components of an organism’s life history—survival versus reproduction, early reproduction versus late reproduction, size versus number of offspring, and so on. The allocation trade-off has since been expanded to include acquisition trade-offs, as described in van Noordwijk and de Jong 1986. Trade-offs can also occur hierarchically. Boggs 2009 provides a framework to predict how allocation patterns are expected to change under different degrees of environmental stress. Allocation trade-offs remain a central concept underlying most life history theory, especially theory based on optimality modeling. Boggs, C. L. 2009. Understanding life histories and senescence through a resource allocation lens. Functional Ecology 23.1: 27–37. Provides a framework for examining interactions among resource acquisition, allocation, and life history traits, and considers the effects of environmental stress on allocation patterns and the magnitude of trade-offs. This general framework can then be applied to understand the phenomenon of senescence (discussed under Aging and Senescence) in insect life histories. Available online for purchase or by subscription. Geber, M. A. 1990. The cost of meristem limitation in Polygonum arenastrum: Negative genetic correlations between fecundity and growth. Evolution 44.4: 799–819. In vascular plants, growth and reproduction both occur via meristems; a meristem can either differentiate vegetatively or produce flowers. This study tests the previously proposed hypothesis that meristem availability should lead to a genetically based trade-off between growth and reproduction. Available online for purchase or by subscription. Stearns, S. C. 1989. Trade-offs in life-history evolution. Functional Ecology 3.3: 259–268. An early review of the concept of a trade-off, and alternative ways to measure trade-offs (for example, at phenotypic versus genetic levels). Students new to this field should read this paper before tackling the other, more challenging papers listed here. Availableonline for purchase or by subscription. van Noordwijk, A. J, and G. de Jong. 1986. Acquisition and allocation of resources: Their influence on variation in life history tactics. American Naturalist 128.1: 137–142. Proposes that variation in resource acquisition explains the frequent inability to detect trade-offs among life history traits. Real trade-offs will be masked if among-individual variation in resource acquisition is large relative to variation in resource allocation; individuals that garner more resources during development may exhibit both higher reproduction and greater longevity. Available online for purchase or by subscription. QUANTITATIVE GENETIC TRADE-OFFS Optimality modeling represents only one approach for understanding trade-offs. Another useful method is the application of quantitative-genetic models to detect and quantify built-in genetic trade-offs (often, negative genetic correlations between fitness traits). This rather large topic is reviewed in Roff 1992 (cited under General Overviews). Roff and Fairbairn 2007 discusses caveats to be considered when studying genetic trade-offs, including that they may arise from, but do not necessarily assume, simple Y-model allocation trade-offs. An advantage of a genetically based evaluation of trade-offs is that evolutionary dynamics can be understood without knowing the underlying physiology. However, this approach is not without problems. For example, genetic correlations do not necessarily reflect pleiotropic genetic effects, but may instead be due to the more transient effects of linkage disequilibrium. In addition, the strength of a genetic correlation between traits depends on the environment in which the correlation is measured; Sgrò and Hoffmann 2004 illustrates how even the sign of the correlation can vary among environments. Genetic correlations may themselves evolve in response to selection, and the genetic correlation is only one of the quantitative-genetic variables that will influence responses to selection. Limitations to the quantitativegenetic approach are outlined in Conner 2012. These problems have led researchers, including Zera and Harshman 2001, to call for more detailed physiological studies of trade-offs, a topic discussed below under Cost of Reproduction by subscription. Conner, J. K. 2012. Quantitative genetic approaches to evolutionary constraint: How useful? Evolution 66.11: 3313–3320. An overview of the concept of a genetic correlation and its consequences for responses to natural selection. The author concludes that genetic correlations have limited value for understanding constraints on life history evolution. Available online for purchase or by subscription. Roff, D. A., and D. J. Fairbairn. 2007. The evolution of trade-offs: where are we? Journal of Evolutionary Biology 20.2: 433–447. Presents important caveats for anyone studying evolutionary trade-offs by focusing on four issues: pitfalls associated with the use of the word “constraint,” the limitations of the aforementioned Y-model, the potential for trade-offs themselves to evolve, and the causes of variation in the magnitude of trade-offs among taxa. Available online for purchase or by subscription. Sgrò, C. M., and A. A. Hoffmann. 2004. Genetic correlations, tradeoffs and environmental variation. Heredity 93:241–248. Review of the evidence that genetic correlations depend on environmental conditions. The authors conclude that genetic correlations between life history traits may be informative or predictive for trade-offs within environments, but are less useful in comparisons among environments. Available online for purchase or by subscription. Zera, A. J., and L. G. Harshman. 2001. The physiology of life history trade-offs in animals. Annual Review of Ecology and Systematics 32:96–126. Stresses the need to establish underlying physiological aspects of life history trade-offs. Considers factors such as nutrient input (and allocation), genetics, and endocrinology. Available by subscription. Evolution in Variable Environments Environmental conditions vary widely across both space and time. This variation commonly leads to evolutionary dynamics that are quite different from those expected in constant environments. Roff 2002 (cited under General overviews) provides a detailed overview of life history evolution in constant versus variable environments, and further contrasts evolutionary responses to environments that vary predictably versus unpredictably (or stochastically). Many organisms can respond to environmental variation by exhibiting different traits in different environments, a phenomenon called phenotypic plasticity. Much plasticity is nonadaptive, but some plasticity reflects evolved responses by which organisms adjust their phenotype (e.g., their growth rate or reproductive strategy) in a manner that increases their fitness. The subject of phenotypic plasticity is far too large and diverse to summarize here. Sultan 2000provides a brief introduction to the concept of plasticity as applied to plant traits, and Whitman and Agrawal 2009 provides a more in-depth introduction to plasticity, focused on insect traits. When environmental variation is predictable, maternal effects can evolve as a mechanism for adaptive plasticity. Here, mothers may use cues in the environment to adjust offspring phenotype to improve fitness in that particular environment. Adaptive maternal effects are a form of transgenerational (or cross-generational) phenotypic plasticity (also called anticipatory maternal effects) and are reviewed by Mousseau and Fox 1998 and Marshall and Uller 2007. Environmental variability (especially temporal variability) has another important consequence for fitness—it causes fitness to vary across generations. Because average fitness across generations is multiplicative rather than additive, it is best described by geometric mean rather than the more familiar arithmetic mean. Geometric mean fitness decreases with increasing temporal variance in fitness. Thus, a trait that minimizes the variation in fitness across generations will outcompete one that may yield the same arithmetic mean fitness but causes greater variation in fitness across generations. Strategies that minimize the variance in fitness are called bet-hedging strategies. Bet-hedging theory is discussed in Childs, et al. 2010; Marshall and Uller 2007 (in the context of maternal effects); and Crean and Marshall 2009 (cited under Withinand Among-Population Variation in Offspring Size). Childs, D. Z., C. J. E. Metcalf, and M. Rees. 2010. Evolutionary bet-hedging in the real world: Empirical evidence and challenges revealed by plants. Proceedings of the Royal Society B 277:3055–3064. Reviews the theory for the evolution of bet-hedging strategies, distinguishing conservative and diversified bet-hedging strategies, and then reviews empirical studies of bet-hedging in a variety of plant systems. Available online for purchase or by subscription. Marshall, D. J., and T. Uller. 2007. When is a maternal effect adaptive? Oikos 116.12: 1957–1963. A discussion of adaptive versus nonadaptive maternal effects. Emphasizes that maternal effects may commonly be adaptive, in that they increase maternal fitness, but that the effects on offspring fitness can be quite variable. This paper also discusses how environmental heterogeneity can favor variance in offspring phenotype as a bet-hedging strategy. Available online for purchase or by subscription. Mousseau, T. A., and C. W. Fox. 1998. The adaptive significance of maternal effects. Trends in Ecology and Evolution 13.10: 403–407. An introduction to how maternal effects are potentially molded by natural selection and provide a mechanism for adaptive responses to environmental variability. Available online for purchase by subscription. Sultan, S. E. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5.12: 537–542. An introduction to the concept of phenotypic plasticity, including discussion of nonadaptive versus adaptive plasticity. Examples are selected from the plant biology literature. Available online for purchase or by subscription. Whitman, D. W., and A. A. Agrawal. 2009. What is plasticity and why is it important? In Phenotypic plasticity of insects: Mechanisms and consequences. Edited by D. W. Whitman and T. N. Ananthakrishnan, 1–63. Enfield, NH: Science Publishers. An overview of the concept of phenotypic plasticity, using examples from the insect biology literature. Reproductive Investment Perhaps the most central topic in the study of life histories concerns reproductive investment, because variation in the amount or schedule of reproductive investment among individuals is a major contributor to variation in fitness. In this section we consider six overlapping aspects of reproductive investment that have received much attention in evolutionary biology. Although sometimes difficult to measure, the cost of reproduction (i.e., the trade-off between current and future reproduction) is a major determinant of variation in life histories. Similarly, selection generates substantial variation in the optimal age or size at which organisms reproduce, whether individuals exhibit single or multiple reproductive bouts, and in the size, number, and spatial dispersion of offspring produced in a given local environment. COST OF REPRODUCTION Much of life history theory is predicated on the assumption of an inescapable trade-off between current reproduction and future reproduction or survival. If resources allocated to current reproduction cannot be allocated to growth or maintenance, higher current reproduction must necessarily reduce future reproduction or survival. Similarly, time allocated to current reproduction cannot be allocated to foraging or vigilance, which might reduce energy intake and increase mortality risk from natural enemies. Recent studies have identified other physiological mechanisms by which current reproduction can affect additional aspects of an organism’s life history; these are reviewed in Harshman and Zera 2007. Several reviews have examined the various types of reproductive costs, the methods used to measure them, and the underlying physiology mechanisms. A few of these are summarized here, and the OBOarticle Reproductive Allocation in Animals by James Gilbert reviews additional examples. One of the major problems with evaluating costs of reproduction is how to define and measure them. Reznick 1985, an immensely influential review (see also Reznick 1992), examined the empirical evidence for costs of reproduction and, more importantly, critiqued the methodology used to measure such costs. The relative merits of the various approaches discussed by Reznick continue to be topics of debate. Flatt 2011 provides a detailed review of survival costs of reproduction, and their underlying physiology, in Drosophila. Harshman and Zera 2007 andEdward and Chapman 2011 review how recent advances in the techniques of animal physiology can help identify underlying reproductive trade-offs in animals. Obeso 2002 reviews the evidence for costs of reproduction in plants and considers issues not covered in the animal literature, such as meristem availability and modularity. Edward, D. A., and T. Chapman. 2011. Mechanisms underlying reproductive trade-offs: Costs of reproduction. In Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Edited by T. Flatt and A. Heyland, 137–152. Oxford: Oxford Univ. Press. A brief review of methods for measuring costs of reproduction, followed by a more detailed review of the physiological mechanisms underlying these costs. Flatt, T. 2011. Survival costs of reproduction in Drosophila. Experimental Gerontology 46.5: 369–375. Presents four general principles for the physiological trade-off between reproduction and life span: it is ubiquitous, can be decoupled in some circumstances, is usually explained by competitive resource allocation, and may or may not reflect genetic trade-offs. Available online for purchase or by subscription. Harshman, L. G., and A. J. Zera. 2007. The cost of reproduction: The devil in the details. Trends in Ecology and Evolution 22.2: 80–86. A review of physiological components underlying costs of reproduction in animals, including metabolic trade-offs, endocrine regulation of life histories, immune function, and physiological stress responses. Available online for purchase or by subscription. Obeso, J. R. 2002. The costs of reproduction in plants. New Phytologist 155.3: 321–348. A review of costs of reproduction in plants. Covers several issues that arise directly from the modularity of plant growth. Availableonline for purchase or by subscription. Reznick, D. N. 1985. Costs of reproduction: An evaluation of the empirical evidence. Oikos 44.2: 257–267. Reviews four methods that had been used to assess costs of reproduction: phenotypic correlations, experimental manipulations, genetic correlations, and correlated responses to artificial selection. Compares the merits of each approach, and argues that too few studies provide evidence for genetically-based reproductive trade-offs. Available online for purchase or by subscription. Reznick, D. N. 1992. Measuring the costs of reproduction. Trends in Ecology and Evolution 7:42–45. Compares two primary methods that have been used to measure reproductive costs: experimental manipulations and genetic correlations. Notes that the mechanisms underlying responses to experimental manipulations can be different from those underlying genetic correlations, and thus that the two methods for identifying costs should not be considered equivalent. Available online for purchase or by subscription. AGE AND SIZE AT REPRODUCTION Maturing earlier carries two benefits: it reduces pre-adult exposure to mortality factors (increasing the probability of surviving to reproduce), and it reduces generation time (which is usually beneficial in a growing population). However, delaying maturation allows additional resources to be allocated to growth, which can substantially increase future reproduction. For example, body size and meristem number commonly increase with age at reproduction, so that late-maturing individuals produce more eggs and seeds. Multiple approaches to understanding age and size at reproduction, and the optimal allocation of resources between growth and reproduction (including cases where organisms can do both simultaneously), were developed starting in the mid-1960s. These studies are too numerous to list here, but Roff 1992, Roff 2002, and Stearns 1992 (all cited under General Overviews) supply general reviews, and Metcalf, et al. 2003 focuses on plant studies. Understanding the fitness consequences of variation in age and size at maturity is at the core of many studies of life history and behavior. Relevant topics include the evolution of sexual size dimorphism, as discussed in Fairbairn, et al. 2009, the evolution of alternative mating tactics, and adaptations to season length. Haselhorst, et al. 2011 demonstrates that resolving the trade-off between optimal size and age at maturity can be environment-specific in plants. Growth rate is an additional dimension of importance; it is usually highly plastic (e.g., in response to food availability). Dmitriew 2011reviews how variation in growth rate necessarily has both immediate (proximate) and longer-term (evolutionary) effects on age or size at maturation Variation in growth rate among different stages is considered by Benesh, et al. 2013 in the specific context of parasites with complex life cycles. Benesh, D. P., J. C. Chubb, and G. A. Parker. 2013. Complex life cycles: Why refrain from growth before reproduction in the adult niche? American Naturalist 181.1: 39–51. Many animals have complex life cycles, in which the various stages can occupy different habitats and use different resources. Here, the authors use life history theory to consider why some parasites restrict growth to particular juvenile stages in productive habitats. Available online for purchase or by subscription. Dmitriew, C. M. 2011. The evolution of growth trajectories: What limits growth rate? Biological Reviews 86.1: 97–116. Considers the evolutionary basis of variation in growth rate, particularly since observed rates are often less than the physiologically maximum (intrinsic) rate. Submaximal growth may be explained by extrinsic factors. For example, the increased feeding that is needed for faster growth might increase predation risk. Available online for purchase or by subscription. Fairbairn, D. J., W. U. Blanckenhorn, and T. Székely. 2009. Sex, size and gender roles: Evolutionary studies of sexual size dimorphism. New York: Oxford Univ. Press. An edited volume examining the diversity of patterns, causes, and consequences of body size dimorphism in animals. Haselhorst, M. S. H, C. E. Edwards, M. J. Rubin, and C. Weinig. 2011. Genetic architecture of life history traits and environment-specific trade-offs. Molecular Ecology 20.19: 4042–4058. Considers the genetic architecture of fitness-related traits and finds a trade-off between age and size at maturity (following the acquisition-allocation model) in a cruciferous plant. However, the magnitude of the trade-off is environment-specific, and thus the degree to which it constrains the evolution of life history traits depends on local conditions. Available online for purchase or by subscription. Metcalf, J. C., K. E. Rose, and M. Rees. 2003. Evolutionary demography of monocarpic perennials. Trends in Ecology and Evolution 18.9: 471–480. Examines evolutionary models and demographic data to predict life history traits such as the size or age of flowering in monocarpic (semelparous) plants, which are more amenable to this type of analysis than polycarpic ones. Available online for purchase or by subscription. PATTERNS OF REPRODUCTION To understand widespread variation in reproductive schedules in nature, Cole 1954 (cited under History) first addressed the simplest dichotomy—whether to reproduce once and die soon after (semelparity), or to spread reproduction among multiple, distinct periods, interspersed with nonreproductive periods (iteroparity). Cole’s analysis led to what became known as Cole’s paradox. Cole showed mathematically that an organism that lives forever producing n offspring per year leaves no more descendants (all else being equal) than one that lives for just one year but produces n+1 offspring. Since producing one more offspring would be a minor cost to many organisms (think of an oak tree producing just one more acorn), it is unclear why iteroparity would ever be evolutionarily stable. Subsequent models, including those of Murphy 1968 and Gadgil and Bossert 1970, resolved this paradox by demonstrating that heterogeneity in reproductive success across reproductive bouts, higher mortality in juveniles than in adults, and density dependent mortality rates could all favor an iteroparous (or polycarpic) strategy. Much subsequent research, discussed in Young 2010, has examined other factors favoring an iteroparous life history. Rather than focus on the causes of differences in parity, Zeineddine and Jansen 2009 considers important evolutionary consequences to this basic difference in reproductive investment. For many organisms, the periods of growth and reproduction are largely distinct, but this need not be the case. Many iteroparous organisms continue to grow after initiating reproduction, and thus must balance allocation between reproduction and growth. The factors that mediate allocation strategies to growth versus reproduction in iteroparous organisms are less well studied than the evolution of iteroparity itself. See Heino and Kaitala 1999 for a review of historically significant papers, and Ejsmond, et al. 2010 for a recent example. How much an iteroparous organism should allocate to any particular reproductive bout has been thoroughly examined in the context of clutch or litter sizes (especially in organisms that cease growth before reproducing), and is discussed in a separate section below. Ejsmond, M. J., M. Czarnołęski, F. Kapustka, and J. Kozłowski. 2010. How to time growth and reproduction during the vegetative season: An evolutionary choice for indeterminate growers in seasonal environments. American Naturalist 175.5: 551–563. A dynamic optimization analysis of resource allocation to growth versus reproduction in a seasonal environment. Available online for purchase or by subscription. Gadgil, M., and W. H. Bossert. 1970. Life historical consequences of natural selection. American Naturalist 104.935: 1–24. One of the earliest mathematical approaches to life history theory, this paper starts with the premise that natural selection modifies how resources are allocated to reproduction to maximize fitness. The model generated testable predictions regarding reproductive effort in the context of variation in age and survivorship schedules. Available online for purchase or by subscription. Heino, M., and V. Kaitala. 1999. Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. Journal of Evolutionary Biology 12.3: 423–429. A review of theory for the optimal allocation of resources to growth versus reproduction in organisms with indeterminate growth and iteroparous reproduction. Though the authors’ main focus is on allocation strategies in animals, the general issues are equally relevant for plants. Available online for purchase or by subscription. Murphy, G. I. 1968. Pattern in life history and the environment. American Naturalist 102.927: 391–403. Perhaps the first paper to consider life history evolution in environments that vary temporally. If the environment imposes high temporal variation in juvenile survival, it “pays” for individuals to live longer and increase the number of reproductive bouts. A precursor to subsequent papers on bet-hedging. Available online for purchase or by subscription. Young, T. P. 2010. Semelparity and iteroparity. Nature Education Knowledge 3.10: 2. A brief overview of the major issues influencing the evolution of semelparous versus iteroparous reproduction, including a bibliography of notable theoretical and empirical studies. Zeineddine, M., and V. A. A. Jansen. 2009. To age, to die: Parity, evolutionary tracking and Cole’s paradox. Evolution 63.6: 1498–1507. Addresses the evolutionary consequences of semelparity versus iteroparity. A mathematical model illustrates that the type of parity influences a population’s evolvability, and hence its ability to track environmental changes (with semelparity increasing the tracking rate of a population). Available online for purchase or by subscription. CLUTCH SIZE IN ITEROPAROUS ORGANISMS Beyond the timing and schedule of reproduction, iteroparous organisms must also “choose” the amount of resources to invest in each reproductive bout. An enormous amount of research has focused on clutch sizes in birds, particularly those with costly altricial young. Modern clutch size theory traces back to Lack 1947 (cited under History). Lack hypothesized that birds with altricial offspring (those that are sedentary and completely dependent on parental care) should produce a clutch of a size that maximizes the number of fledglings that survive to reproduce (Lack subsequently extended this idea to mammal litters). This “most productive clutch,” also known as the “Lack clutch size,” reflects a balance between the number of eggs laid and the ability of parents to feed the hatchlings. However, experimental studies, initially reviewed in Cody 1966, consistently demonstrated that birds lay fewer eggs per clutch than they are capable of fledging; that is, observed clutches were typically smaller than Lack clutch size. Williams 1966 noted that predictions of optimal clutch size need to consider lifetime reproductive success of the parent, rather than just the current most-productive clutch size. Williams 1966, and subsequent research, including Charnov and Krebs 1974, indicated that trade-offs with other aspects of the life history, such as future reproduction, could favor smaller clutches. Godfray, et al. 1991 reviewed evidence that larger current-generation clutches increase the risk of predation during parenting or reduce overwinter survival. Several other hypotheses have since been proposed to explain why observed clutch sizes do not match the most productive one, including effects of cannibalism, within- and between-season variation in environmental quality (and hence juvenile survival), individual optimization of clutch size based on body condition, and the asymmetrical penalties associated with laying too few versus too many eggs (“the cliff-edge effect”). These issues are summarized in Boyce and Perrins 1987 and Vercken, et al. 2012. Lloyd 1980 and Obeso 2002 (cited under Cost of Reproduction) discuss the analogous question of how many seeds should be produced in a given reproductive bout by iteroparous plants, and Sibly, et al. 2012 identifies phylogenetic constraints on reproductive investment in birds. Boyce, M. S., and C. M. Perrins. 1987. Optimizing great tit clutch size in a fluctuating environment. Ecology 68.1: 142–153. Argues that geometric mean fitness across years will be maximized by laying fewer eggs than the most productive clutch size if (1) environmental variability causes variance in the optimal clutch size among years, and (2) the penalty for exceeding the Lack clutch size is more severe than for laying too few eggs. Average fitness is calculated using the geometric mean rather than the arithmetic mean because mean fitness across generations is multiplicative rather than additive. Available online for purchase or by subscription. Charnov, E. L., and J. R. Krebs. 1974. On clutch-size and fitness. Ibis 116.2: 217–219. An early formal analysis demonstrating how effects of reproductive effort on survival in an iteroparous organism could account for observed clutch sizes below the Lack clutch size. The authors’ framework emphasized that, in the absence of concomitant data on adult mortality, egg-addition experiments are not sufficient to estimate whether observed clutch sizes are submaximal with respect to overall fitness. Available online for purchase or by subscription. Cody, M. L. 1966. A general theory of clutch size. Evolution 20.2: 174–184. An influential early paper that systematically analyzes all the hypotheses proposed before 1966 to explain variation in clutch size, including now-discarded and untenable group-selection explanations (i.e., explanations in which traits evolve by virtue of their advantage to a group of organisms, despite entailing significant costs to the individual expressing the trait). This paper set the tone for later reviews by using available data to examine effects of such factors as latitude, insularity, and predation risk. Available onlinefor purchase or by subscription. Godfray, H. C. J., L. Partridge, and P. H. Harvey. 1991. Clutch size. Annual Review of Ecology and Systematics 22:409–429. A review of clutch size evolution that, unlike earlier reviews, is not excessively focused on vertebrates. The paper also describes improvements in various approaches since the early ornithological work, including more refined experimental manipulations, more rigorous and unbiased analyses of comparisons among taxa, and the incorporation of more quantitative-genetic data. Availableonline for purchase or by subscription. Lloyd, D. G. 1980. Sexual strategies in plants. I: An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytologist 86.1: 69–79. Outlines how angiosperms optimize reproductive effort by making “adjustments” in maternal expenditures at different stages: flower determination, ovary development, and fruit maturation. Lloyd emphasizes the adaptive value of plasticity in resource allocation, and documents how different species vary in the proportional use of the three stages to adjust expenditures. Available online for purchase or by subscription. Sibly, R. M, C. C. Witt, N. A. Wright, C. Venditti, W. Jetz, and J. H. Brown. 2012. Energetics, lifestyle, and reproduction in birds.Proceedings of the National Academy of Sciences 109.27: 10937–10941. Explores variation in reproductive output in birds. A large dataset (980 species) reveals how reproduction is influenced by factors such as parental care and migration. Importantly, the authors find a strong phylogenetic signal, suggesting that life history trait combinations tend to be conserved in higher taxa. Available online for purchase or by subscription. Vercken, E., M. Wellenreuther, E. Svensson, and B. Mauroy. 2012. Don’t fall of the adaptation cliff: When asymmetrical fitness selects for suboptimal traits. PLOS One 7.4: e34889. A recent extension of the cliff-edge hypothesis considered by Boyce and Perrins 1987. Provides a general model that can be applied to any asymmetric fitness function, in which the evolutionarily stable strategy depends on the shape of the function and its variance. Williams, G. C. 1966. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. American Naturalist100.916: 687–690. A formal analysis of survival costs associated with different amounts of reproductive effort. Notes the importance of an organism’s residual reproductive value. Whereas Lack mainly focused on explaining within-species variation in clutch size, Williams urged comparisons across species that differ in residual reproductive value. Available online for purchase or by subscription. OFFSPRING SIZE The first formal analysis of optimal progeny size was given in Vance 1973, but the most influential treatment was Smith and Fretwell 1974, one of the most cited papers in the history of ecology. Smith and Fretwell’s model illustrated a couple of important points. For any fixed allocation of resources to reproduction, offspring size will be under stabilizing selection, with the optimal size depending on the shape of the relationship between offspring size and offspring fitness. Second, there will be a conflict of interests between parents and offspring—the offspring size that maximizes individual maternal fitness is not the same that maximizes offspring fitness. A diversity of subsequent models relaxed many of the assumptions of the Smith-Fretwell model, but the main conclusions first noted by Smith and Fretwell remain intact. Experimental tests of the assumptions and predictions of the Smith-Fretwell model are reviewed in Fox and Czesak 2000. The ecological variables that mediate selection on offspring size, such as dispersal, competition, and environmental quality, have been especially well studied in plants, as reviewed in Moles and Westoby 2004. The assumptions of the Smith-Fretwell model are simple, which gives the model a high degree of generality. However, if the assumptions of the model are too simplistic, the model may fail to predict some common patterns. For example, the Smith-Fretwell model confounds the fitness of offspring and parents; it assigns fitness (survival) of offspring to parents. Wolf and Wade 2001 shows how viewing fitness in this way can lead to incorrect conclusions about the overall effect of selection and the direction of evolution. Rollinson and Hutchings 2013provides a discussion of empirical and statistical issues in the measurement of offspring fitness curves (the relationship between offspring size and fitness). Smith-Fretwell also assumes that the amount of resources allocated to individual progeny is invariant (e.g., does not depend on fecundity), does not itself evolve, and hence does not affect optimal offspring size. In real organisms, offspring allocation to reproduction can evolve rapidly in response to selection on offspring size. Czesak and Fox 2003 provide an example of how the trade-off between offspring size and number in an insect can depend on the environment in which females reproduce. Czesak, M. E., and C. W. Fox. 2003. Evolutionary ecology of egg size and number in a seed beetle: Genetic trade-off differs between environments. Evolution 57.5: 1121–1132. Examines how the trade-off between egg size and number in a beetle depends on the environment (the beetle’s host plant). Also, egg size was genetically correlated with female mass on one host, which contradicts the assumption of life history theory that total reproductive effort and egg size can evolve independently. Available online for purchase or by subscription. Fox, C. W., and M. E. Czesak. 2000. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology 45:341–369. Although it focuses on arthropods, this review examines general conditions under which the egg size-number tradeoff is expected to be relatively strong or weak. The paper also considers the robustness of the assumption that fitness will be positively related to progeny size, and calls for more empirical data. Available online for purchase or by subscription. Moles, A. T., and M. Westoby. 2004. What do seedlings die from and what are the implications for evolution of seed size? Oikos106.1: 193–199. Compiles a large dataset to examine the assumption that larger seeds have an increased probability of establishment. Moreover, the authors are able to delineate the particular seedling stage at which size variation is most important, and they examine the advantage of large seed size in stressful environments, including shade, drought, and herbivory. Available online for purchase or by subscription. Rollinson, N., and J. A. Hutchings. 2013. The relationship between offspring size and fitness: Integrating theory and empiricism.Ecology 94.2: 315–324. An evaluation of what measures of fitness, and which statistical techniques, should be used to quantify the relationship between offspring size and fitness. Available online for purchase or by subscription. Smith, C. C., and S. D. Fretwell. 1974. The optimal balance between size and number of offspring. American Naturalist 108. 962: 499–506. Calculates the progeny size at which a female maximizes her number of grandprogeny. The solution depends on both the number of offspring produced and the probability that offspring survive to reproductive age. Available online for purchase or by subscription. Vance, R. R. 1973. On reproductive strategies in marine benthic invertebrates. American Naturalist 107.955: 339–352. Models the evolution of egg size in marine invertebrates to understand why some planktonic larvae suppress feeding and depend on abundant yolk for development, whereas other larvae need to feed as larvae or are brooded by adults. The results contrast with the prediction of Smith and Fretwell 1974. Available online for purchase or by subscription. Wolf, J. B., and M. J. Wade. 2001. On the assignment of fitness to parents and offspring: Whose fitness is it and when does it matter? Journal of Evolutionary Biology 14.2: 347–356. Shows how assignment of offspring fitness (early survival) directly to mothers (as a component of maternal fitness), as opposed to the offspring themselves, affects both the magnitude of selection on traits (such as egg size) and the evolutionary dynamics of those traits. Available online for purchase or by subscription. Within- and Among-Population Variation in Offspring Size Offspring size also commonly varies among females, often depending on maternal size or resource status. The maternal size–offspring size relationship is commonly assumed to be nonadaptive, and simply the result of constraints. Sakai and Harada 2001and Kindsvater, et al. 2011 examine the evolution of among-individual variation in offspring size. Offspring size can also vary substantially within a family (e.g., within individual broods). Marshall, et al. 2008 examines how unpredictable variation in environmental quality can favor producing offspring that vary in size, even within a single brood. Crean and Marshall 2009 further illustrates how this tactic may be yet another example of bet-hedging in life histories (see Evolution in Variable Environments). Offspring size also varies with the environmental conditions experienced by females; that is, it is highly plastic. Much of this variation is likely nonadaptive, but some may reflect an adaptive, plastic response to resource quality, as reviewed in Fox and Czesak 2000(cited under Offspring Size). Plasticity of offspring size in response to temperature is common—offspring are often larger when their parents develop at lower temperatures—but Fischer, et al. 2003 suggests the evidence that this plasticity is adaptive is inconsistent. Plastic variation in offspring size can have substantial consequences for population dynamics, since it affects the likelihood of density-dependent population growth, as described in Plaistow, et al. 2007. Crean, A. J., and D. J. Marshall. 2009. Coping with environmental uncertainty: Dynamic bet hedging as a maternal effect.Philosophical Transactions of the Royal Society B 364:1087–1096. A review of the role of bet-hedging in the evolution of within-female variation in offspring size, with four case studies examining the empirical evidence for maternal bet-hedging in response to environmental unpredictability. Available online for purchase or by subscription. Fischer, K., P. M. Brakefield, and B. J. Zwaan. 2003. Plasticity in butterfly egg size: Why larger offspring at lower temperatures?Ecology 84.12: 3138–3147. An experimental study of the fitness consequences of temperature-mediated plasticity in egg size in the butterfly Bicyclus anynana. Data suggest that larger eggs are favored (albeit weakly) by selection at lower temperatures. Available online for purchase or by subscription. Kindsvater, H. K., M. B. Bonsall, and S. H. Alonzo. 2011. Survival costs of reproduction predict agedependent variation in maternal investment. Journal of Evolutionary Biology 24.10: 2230–2240. Uses dynamic state-variable models to explain variation in offspring size according to the age, condition, and size of a female, and the survival cost of reproduction. Optimal offspring size depends on a complex interaction among these variables. The model can predict when we should see deviations from Smith-Fretwell predictions. Available online for purchase or by subscription. Marshall, D. J., R. Bonduriansky, and L. F. Bussiere. 2008. Offspring size variation within broods as a bethedging strategy in unpredictable environments. Ecology 89.9: 2506–2517. Considers adaptive hypotheses for variation in offspring size within a single brood. The authors’ model and review of empirical evidence suggest producing offspring that vary in size increases mean fitness (and decreases variation in fitness) if environments vary unpredictably in quality. Hence selection can act on the variance in offspring size in addition to the mean. Available online for purchase or by subscription. Plaistow, S. J, J. J. H. St. Clair, J. Grant, and T. G. Benton. 2007. How to put all your eggs in one basket: Empirical patterns of offspring provisioning throughout a mother’s lifetime. American Naturalist 170.4: 520– 529. An empirical study with mites (Sancassania berlesei) demonstrating that maternal allocation strategies (offspring size versus number) vary with female age, and that this change is likely an adaptive response to changing density associated with rapid population growth. Available online for purchase or by subscription. Sakai, S., and Y. Harada. 2001. Why do large mothers produce large offspring? Theory and a test. American Naturalist 157.3: 348–359. Examines the common observation that larger females tend to produce larger offspring, which is not predicted by the Smith-Fretwell model. Their model postulates that the rate at which resources can be transported to offspring (seeds) is limited, and depends on maternal size. OFFSPRING DISPERSION A large proportion of animal species must distribute their offspring (eggs) among many discrete patches, such as the widely scattered food sources of parasitic and herbivorous insects. In many of these species, juveniles must complete development in a single host or patch selected by their mothers. Mangel 1987 noted that producing the optimal distribution of offspring among available hosts thus becomes a problem in foraging theory. Hypotheses developed to explain how iteroparous organisms should partition eggs among distinct reproductive bouts can be applied to how semelparous (or at least short-lived) females should spread their eggs among discrete hosts. Charnov and Skinner 1985 argued that the optimal number of eggs to deposit per host can be viewed (and modeled) as analogous to producing the optimal clutch size during a given breeding season. Clutch sizes can be adaptively adjusted according to variation in host quality, which includes whether a host is already occupied by potential competitors. A key concept, put forth in Ives 1989, is that optimal clutch size will depend on the larval competition curve, which describes how the combined fitness of larvae sharing a patch or host changes with increasing larval density. The equivalent to the Lack clutch size in birds is typically called the single-host maximum, or the number of eggs that would maximize total productivity from a single host or patch. Like nesting birds, female insects frequently deposit fewer eggs than the single-host maximum, and much research has sought to explain this discrepancy. For example, female decisionmaking is expected to depend on search costs, which affect the rate of fitness gain she would achieve by adding more eggs to a current host versus searching for a new one. Most of these models assume that a searching female’s reproductive success is limited by the time available to locate suitable hosts. Rosenheim 2011reviews the evidence that a female is instead egg-limited. Under egg limitation, adding more eggs to a clutch could entail an opportunity cost; an egg-depleted female loses the opportunity to exploit a higher-quality host that might be encountered later. It has been difficult to distinguish between time limitation and egg limitation under natural conditions; the frequency of egg limitation will likely depend on the female’s expected mortality rate and ovarian dynamics (e.g., her rates of egg maturation, deposition, and possible resorption). Papaj 2000 explicitly considers the effects of host quality and availability on ovarian dynamics in insects. In the face of trade-offs between egg production and survival or dispersal ability, as well as short-term changes in host availability, reproductive effort can be adaptively adjusted in the upward or downward direction. Charnov, E. L., and S. W. Skinner. 1985. Complementary approaches to the understanding of parasitoid oviposition decisions.Environmental Entomology 14.4: 383–391. Extends the concept of the Lack clutch size to ovipositing insects, and discusses reasons why female fitness is maximized if she lays fewer eggs on a host than the single-host maximum. Ives, A. R. 1989. The optimal clutch size of insects when many females oviposit per patch. American Naturalist 133.5: 671–687. Considers how the optimal clutch size should be modified if multiple females are likely to oviposit on the same patch or host. The answer to this question depends mainly on the curve describing the combined fitness of all larvae (or total productivity of the patch) as a function of egg or larval density. Available online for purchase or by subscription. Mangel, M. 1987. Oviposition site selection and clutch size in insects. Journal of Mathematical Biology 25.1: 1–22. Because a female’s physiological state changes with successive visits to multiple hosts, dynamic-optimization models provide a refinement to static models, and predict how a female’s clutch size will depend on several state variables, such as a female’s age, current egg load, and recent experience. Available online for purchase or by subscription. Papaj, D. R. 2000. Ovarian dynamics and host use. Annual Review of Entomology 45:423–448. Considers plastic responses in egg production among herbivorous and parasitic insects that must scatter their eggs among multiple hosts. This comprehensive review uses both physiological and ecological approaches to understand how these insects can track changes in resource availability. Available online for purchase or by subscription. Rosenheim, J. A. 2011. Stochasticity in reproductive opportunity and the evolution of egg limitation in insects. Evolution 65.8: 2300–2312. A reassessment of the debate over whether egg-laying females are primarily time- or egg-limited in terms of reproductive success. Rosenheim introduces a model that can be used to predict the likelihood of each type of limitation when environments (and reproductive opportunities) vary stochastically. Available online for purchase or by subscription. Dispersal Dispersal is the movement of organisms over the course of their lifetimes, with the important consequence that they reproduce in a different location from where they were born, or at least in a different location from the one used for a previous reproductive bout. Dispersal affects local population sizes and thus mediates density-dependent rates of births and deaths. Dispersal is important in life history analysis because it too entails trade-offs; the need to disperse typically delays reproduction and often requires substantial time and resources that could otherwise be allocated to reproduction. Dispersal also mediates gene flow, which can prevent or slow down local adaptation. Dispersal has thus been of longstanding interest to researchers in population genetics and ecology. The diversity of theoretical and empirical advances is too great to summarize here, so the coverage here is limited to significant reviews. For a recent synthesis of research on dispersal, see Bonte, et al. 2012 and the edited volume Clobert, et al. 2012. Johnson and Gaines 1990 considers early theoretical models for the evolution of dispersal. The ecology of metapopulations— populations distributed throughout a fragmented landscape but connected by movement (dispersal) of individuals among fragments—is reviewed in Hanski 1999. Sex-biased dispersal, a common phenomenon that is especially well studied in mammals, is reviewed inHandley and Perrin 2007. Zera and Denno 1997 reviews life history trade-offs associated with dispersal in species with dispersal polymorphisms (best studied in insects, but found in many other organisms, including plants with heteromorphic seeds). A recent special feature in PNAS (Nathan, et al. 2008) examines the ecology of movement, including dispersal. Philips, et al. 2010 examine the role of dispersal in mediating range expansions of species, and how range expansion affects life history evolution, a topic that is especially timely in the face of human-mediated environmental change. The prevalence and significance of long-distance seed dispersal is discussed in Cain, et al. 2000. Bonte, D., H. van Dyck, J. M. Bullock, et al. 2012. Costs of dispersal. Biological Reviews 87:290–312. A review of the energetic, time, risk and opportunity costs associated with dispersal in microbes, plants, invertebrates and vertebrates. Available online for purchase or by subscription. Cain, M. L., B. G. Milligan, and A. E. Strand. 2000. Long-distance seed dispersal in plant populations. American Journal of Botany87.9: 1217–1227. Argues that long-distance dispersal of seeds may be hard to measure but is critical for understanding such phenomena as the invasiveness of some exotic plants, the connectedness of fragmented plant populations, or the colonization of islands. Availableonline for purchase or by subscription. Clobert, J., M. Baguette, T. G. Benton, and J. M. Bullock, eds. 2012. Dispersal Ecology and Evolution. Oxford: Oxford Univ. Press. Comprehensive overview of the importance of dispersal from the perspective of both evolution and ecology; considers both the causes and consequences of variation on dispersal behavior, including its relationship to other life history traits. Johnson, M. L., and M. S. Gaines. 1990. Evolution of dispersal: Theoretical models and empirical tests using birds and mammals.Annual Review of Ecology and Systematics 21:449–480 Reviews a wide range of modeling techniques aimed at understanding the evolution of dispersal (including sexbiased dispersal) and its consequences, and explores the role of environmental variability (both temporal and spatial) in determining the optimal dispersal rate. Available online for purchase or by subscription. Lawson Handley, L. J., and N. Perrin. 2007. Advances in our understanding of mammalian sex-biased dispersal. Molecular Ecology 16.8: 1559–1578. Examines the proximate causes and evolutionary bases of sex-biased dispersal. Argues that the direction of bias (female vs. male) is not merely a function of a species’ mating system. Calls for more attention to complex interactions among causative factors such as inbreeding avoidance, competition between kin, and cooperation between kin. Available online for purchase or by subscription. Hanski, I. 1999. Metapopulation Ecology. Oxford: Oxford Univ. Press. An early and influential synthesis of models and empirical data addressing the dynamics of organisms distributed in discrete patches with varying degrees of connectedness. The book is especially useful for the study of fragmented populations in the context of conservation biology. Nathan, R., W. Getz, E. Revilla, et al. 2008. Movement Ecology Special Feature: A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences 105.49: 19052–19059. Introduces a journal special feature on the ecology of movement that addresses common themes in the study of dispersal. Availableonline for purchase or by subscription. Philips, B. L., G. P. Brown, and R. Shine. 2010. Life-history evolution in range-shifting populations. Ecology 91.6: 1617–1627. A discussion of how range expansion mediates life history evolution. Available online for purchase or by subscription. Zera, A. J., and R. F. Denno. 1997. Physiology and ecology of dispersal polymorphism in insects. Annual Review of Entomology42:207–230. Uses wing-dimorphic insects to examine the role of habitat persistence in the tendency to disperse, and documents the common trade-off between dispersal and reproduction. The authors suggest that these ecological and evolutionary approaches should be complemented by more information on the proximate, physiological mechanisms underlying wing-morph determination. Availableonline for purchase or by subscription. MIGRATION The term migration is used interchangeably with dispersal in the population genetics literature, and it is used by biogeographers to describe range expansions of faunas or individual species. In ecology, migration is more commonly used to describe directional (and often coordinated) movements of individuals, often over great distances, with a subsequent return movement either within a generation (most migrating vertebrate animals) or in a subsequent generation (e.g., successive generations of monarch butterflies). Migration has evolved independently in a wide diversity of animal taxa, and is commonly a response to seasonal or habitat deterioration. Dingle and Drake 2007 outlines a general framework for classifying types of migration. Alerstam, et al. 2003 reviews long-distance migration and discusses both the evolution of migration—the environmental factors that favor migration and the life history costs that balance these benefits—and the behavioral and physiological adaptations found in migratory animals. A more detailed review of migration biology—its causes and consequences—is presented in Milner-Gulland, et al. 2011. Alerstam, T., A. Hedenstrom, and S. Akesson. 2003. Long-distance migration: Evolution and determinants. Oikos 103.2: 247–260. A review of the ecology of migration in animals, including the ecological factors favoring migration, the mortality and physiological costs of migration, and mechanisms of migration. Available online for purchase or by subscription. Milner-Gulland, E. J., J. M. Fryxell, and A. R. E. Sinclaire. 2011. Animal migration: A synthesis. Oxford: Oxford Univ. Press. An edited volume that reviews the evolution of migration, the behavioral and physiological mechanisms facilitating migration, the evolution of migratory life histories, and the conservation and management implications. Dingle, H., and A. Drake. 2007. What is migration? BioScience 57.2: 113–121. Reviews various conceptual issues in the study of migration, including the various meanings of the word migration, migratory behavior of migratory individuals, and the evolutionary and ecological consequences of migration (e.g., for population dynamics). Available online for purchase or by subscription. Sex Ratios and Sex Allocation One of the triumphs of life history theory is explaining observed variation in sex ratios. Simple models of sex ratio evolution commonly predict observed sex ratios, in part because the “decision” here is relatively straightforward— offspring must be either male or female (except in the case of hermaphrodites). The question of why a 1:1 ratio is common in nature was famously discussed by Darwin, and was subsequently the topic of substantial theoretical debate. A solution was first presented in Fisher 1930. Fisher noted that, because every offspring has both a mother and a father, both sexes contribute gametes equally to the next generation, and thus must have equal fitness. If the population sex ratio deviates from 1:1, individuals of the rare sex will have greater fitness than will individuals of the common sex. Thus, if there is genetic variation among individuals for the sex ratio of their offspring, there will be frequency-dependent selection: genotypes that produce more offspring of the sex that is currently rare will be favored until the population arrives at an even sex ratio, after which neither sex has a fitness advantage. Fisher’s verbal argument was formalized mathematically in Shaw and Mohler 1953. Testing this theory initially proved complicated because a 1:1 sex ratio is the null expectation in organisms where sex is determined by chromosomal inheritance, as in many animals. However, genetic mechanisms of sex determination vary considerably among species (and some species rely on environmental sex determination), and sex ratio theory appears to apply equally well in these species with alternative forms of sex determination. A particularly elegant test of sex ratio theory is presented in Basolo 1994. Basolo, A. L. 1994. The dynamics of Fisherian sex-ratio evolution: Theoretical and experimental investigations. American Naturalist 144.3: 473–490. In the platyfish (Xiphophorus maculatus), sex is determined by a single locus with three alleles, and sex ratio is thus not constrained to be equal males and females by the mechanism of sex determination. Experimental perturbations of sex ratio demonstrate that (a) the rare gender is indeed favored by frequency-dependent selection, and (b) population sex ratios quickly evolve back to an equal sex ratio. Available online for purchase or by subscription. Fisher, R. A. 1930. The genetical theory of natural selection. Oxford: Clarendon. A landmark book for nearly all of evolutionary biology. It provides a verbal explanation for why populations so commonly converge on a 1:1 sex ratio, regardless of the specific sex-determination mechanism. Shaw, R. F., and J. D. Mohler. 1953. The selective significance of the sex ratio. American Naturalist 87.837: 337–342. Models the evolution of sex ratio and concludes that 1:1 sex ratios should evolve via frequency-dependent selection favoring the rare sex, as conjectured by Fisher 1930. Also postulates that selection can favor deviations from 1:1 sex ratios, but does not explore the conditions that would maintain unequal sex ratios. Available online for purchase or by subscription. DEVIATION FROM 1:1 SEX RATIOS Basic sex ratio theory makes two important assumptions that are commonly violated—that the population is panmictic (mating is random), and that allocation of resources is equal to male and female offspring. These violations lead to predictions of when sex ratios will vary from 1:1, either slightly or considerably. Indeed, unequal sex ratios may not be as rare as was thought initially, and they can occur in species with simple chromosomal sex determination. Deviations from an equal sex ratio are well understood in a few cases. The theoretical issues, and empirical tests, are reviewed in West, et al. 2005 and West 2009. West, S. A. 2009. Sex allocation. Princeton, NJ: Princeton Univ. Press. Synthesizes the literature on sex allocation (including sex ratio) by placing empirical observations into a conceptual framework and highlighting areas where inconsistencies and controversies remain. West, S. A., D. M. Shuker, and B. C. Sheldon. 2005. Sex-ratio adjustment when relatives interact: A test of constraints on adaptation. Evolution 59.6: 1211–1228. Uses a meta-analysis to examine how the mechanism of sex determination affects sex ratio adjustment in response to local resource competition, local mate competition, and local resource enhancement. Concludes that theory needs to account for variation in the specific means of sex determination. Available online for purchase or by subscription. Nonrandom Mating The effect of nonrandom mating on sex ratios was originally explored in Hamilton 1967, which showed that femalebiased sex ratios are favored in situations where the environment is divided into many spatially segregated mating groups, and in which individuals mate before dispersing. If the offspring in a group are the result of multiple foundresses (so offspring are not all closely related), a more balanced sex ratio is expected. However, in the extreme case where each group is founded by a single female, the equilibrium sex ratio should include only enough male offspring to fertilize all female offspring. This local mate competition model has been extensively examined theoretically (e.g., Taylor and Bulmer 1980), and widely tested empirically (e.g., in Werren 1980 and Macke, et al. 2011). Other models consider how other interactions among relatives will affect sex ratios; for example, Clark 1978 explores the effect on sex ratio evolution of local resource competition, and Emlen, et al. 1986 examines the effect of local resource enhancement. Model predictions are commonly supported by empirical studies. For example, Griffin, et al. 2005 finds that there is greater sex ratio adjustment in cooperatively breeding birds and mammals when helpers provide larger benefits, and Silk and Brown 2008 finds that sex-ratio skew in primates fits the predictions of local resource enhancement models. Clark, A. B. 1978. Sex ratio and local resource competition in a prosimian primate. Science 201.435: 163–165. Proposed the hypothesis that sex ratio should be biased toward the dispersing sex when offspring compete for resources with their mother. Available online for purchase or by subscription. Emlen, S. T., J. M. Emlen, and S. A. Levin. 1986. Sex ratio selection in species with helpers-at-thenest. American Naturalist127.1: 1–8. Builds on published observations that sex ratios commonly deviate from 1:1 in cooperatively breeding birds, and proposes that selection should favor parents to skew offspring sex toward the sex that is the better “helper,” because helpers repay part of the cost of their production. Available online for purchase or by subscription. Griffin, A. S., B. C. Sheldon, and S. A. West. 2005. Cooperative breeders adjust offspring sex ratios to produce helpful helpers.American Naturalist 166.5: 628–632. A meta-analysis of whether cooperatively breeding birds and mammals adjust their offspring sex ratios adaptively in response to their environment. The authors conclude that there is greater sex ratio adjustment when helpers bring larger benefits. Availableonline for purchase or by subscription. Hamilton, W. D. 1967. Extraordinary sex ratios: A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156.3774: 477–488. Models how deviations from the assumptions made by Fisher affect the evolution of sex ratios. Notably, the paper examines how competition among relatives for mates affects the optimal sex ratio, giving rise to the influential “local mate competition” hypothesis. Available online for purchase or by subscription. Macke, E., S. Magalhaes, F. Bach, and I. Olivieri. 2011. Experimental evolution of reduced sex ratio adjustment under local mate competition. Science 334.6059: 1127–1129. Experimentally manipulated the number of foundresses in replicate mating groups and tracked the evolution of sex ratio over fifty four generations in a haplodiploid spider mite. Females responded to local mate competition as predicted by theory. Available online for purchase or by subscription. Silk, J. B., and G. R. Brown. 2008. Local resource competition and local resource enhancement shape primate birth sex ratios.Proceedings of the Royal Society B 275.1644: 1761–1765. Found that sex ratio is skewed in favor of the dispersing sex in primate species that do not breed cooperatively, and skewed in favor of the more beneficial sex in cooperatively breeding species, as predicted by the local resource competition and local resource enhancement models, respectively. Available online for purchase or by subscription. Taylor, P. D., and M. G. Bulmer. 1980. Local mate competition and sex ratio. Journal of Theoretical Biology 86.3: 409–419. A detailed theoretical examination of the local mate competition hypothesis originally proposed in Hamilton 1967. Available online for purchase or by subscription. Werren, J. H. 1980. Sex ratio adaptations to local mate competition in a parasitic wasp. Science 208.4448: 1157–1158. Female Hymenoptera (wasps, bees, and ants) can control sex ratio by manipulating whether eggs are fertilized (and become females) or not (and become males). In this paper and a subsequent one (“Sex Ratio Evolution under Local Mate Competition in a Parasitic Wasp,” Evolution 37.1 [1983]: 116–124) female behavior conforms to predictions of optimal sex ratios if there is local mate competition. Available online for purchase or by subscription. Responses to Environmental Quality In some species, females adjust sex ratio in response to environmental conditions that differentially affect the fitness of male versus female offspring. This situation was first modeled for species providing parental care (Trivers and Willard 1972) and for parasitic Hymenoptera (Charnov 1979). Trivers and Willard predicted that parents should bias the sex ratio of their offspring toward sons when they are in good condition, and toward daughters when in poor condition. This will hold when maternal condition affects offspring condition, and being in good condition benefits males more than females. This hypothesis has mixed support from human populations but is supported by some data in nonhuman species. Charnov 1979 showed that sex ratio of progeny should vary with host size in parasitic wasps. More daughters should be produced for large hosts because offspring can grow to be larger on large hosts, and the marginal gain in fitness with increasing body size is greater for females than for males. Ode and Hunter 2002 shows that this prediction is widely supported in empirical studies of Hymenoptera. An analogous situation occurs in many organisms in which sex is environmentally determined (best studied in reptiles and fish with temperature-dependent sex determination). Some experimental evidence, such as that presented in Warner and Shine 2008, indicates that embryos develop into males when incubated under conditions that promote high fitness for males, and likewise for females. Janzen and Phillips 2006 presents a critical review of this topic. In addition, the effect of the environment on sex ratio (and the switch between environmental and genetic sex determination) can evolve in response to environmental change, as demonstrated in Pen, et al. 2010. Charnov, E. L. 1979. The genetical evolution of patterns of sexuality: Darwinian fitness. American Naturalist 113.4: 465–480. An early theoretical exploration the evolution of sex ratios that specifically examines the choice of sexual state (hermaphroditism versus dioecy) and allocation to the male versus female offspring in parasitic Hymenoptera. Available online for purchase or by subscription. Janzen, F. J., and P. C. Phillips. 2006. Exploring the evolution of environmental sex determination, especially in reptiles. Journal of Evolutionary Biology 19.6: 1775–1784. Reviews the evolution of environmentally based sex determination and its consequences for sex-ratio evolution. Available online for purchase or by subscription. Ode, P. J., and M. S. Hunter. 2002. Sex ratios of parasitic Hymenoptera with unusual life-histories. In Sex Ratios: Concepts and Research Methods. Edited by I. C. W. Hardy, 218–234. Cambridge, UK: Cambridge Univ. Press. A review of sex allocation strategies of parasitic Hymenoptera. Parasitic Hymenoptera are particular useful models because females lay eggs on discrete resources (e.g., other insects, fruits, or seeds) that can be easily manipulated to vary in size or other measures of quality. Pen, I., T. Uller, B. Feldmeyer, A. Harts, G. M. While, and E. Wapstra 2010. Climate-driven population divergence in sex-determining systems. Nature 468:436–438. Demonstrates experimentally that there is geographic variation in the sex-determination mechanism in a lizard, and that the variation in sex-determination mechanism and sex ratios is as predicted by selection. Available online for purchase or by subscription. Trivers, R. L., and D. E. Willard. 1972. Natural selection of parental ability to vary the sex ratio of offspring. Science 179.4068: 90–92. The original model that predicts parents will bias the sex ratio of their offspring toward sons when they are in good condition and toward daughters when in poor condition. Available online for purchase or by subscription. Warner, D. A., and R. Shine. 2008. The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451 (31 January): 566–568. A test of the Charnov–Bull model for the adaptive significance of temperature-dependent sex determination (reviewed in Janzen and Phillips 2006). Incubation temperature affects reproductive success of males differently than females in a lizard, and the fitness of each sex was maximized by the incubation temperature that produces that sex. Available online for purchase or by subscription. Hermaphroditism and Dioecy Though most plant species are monoecious (both sexes are found in the same individual), about 7 percent are dioecious (individuals are one sex or the other). The origins of dioecy, its maintenance in populations, and its consequences for the evolution of life history traits have been the subject of substantial interest at least since Darwin’s generation. Charlesworth 1999 provides a review of the origin and maintenance of dioecy. Barrett, et al. 2010 reviews sex ratios in plants, which often differ from 1:1, with male-bias more common than female-bias. Barrett and Hough 2013 reviews the evolution of sexual dimorphism in dioecious plants, particularly a scenario in which differences between sex morphs reduce between-sex competition for resources. Jacobs and Wade 2003 reviews theoretical issues in the evolution of gynodioecy, the situation in which populations include both females and hermaphrodites. Gynodioecy is common in plants, but androdioecy, in which populations consist of males and hermaphrodites, is rare. Hermaphroditism is less common in animals. A particularly interesting case is that of sequential hermaphroditism, in which individuals can change sex. Changing sex can be adaptive when the relationship between size or age and fitness differs between the sexes. The optimal switch point was investigated theoretically by Warner 1975, based on Ghiselin’s size-advantage model (Ghiselin 1969). The basic intuition from these models is that individuals should change sex when the male and female fitness functions (fitness versus size or age) cross. For example, because seeds are more expensive than pollen, the advantages of large size are especially high for females in some sequentially hermaphroditic plants. It therefore “pays” for an individual to reproduce as a male when it is young, small, or stressed, but switch to female at the age or size when the female fitness function exceeds that of the male (Freeman, et al. 1980). In contrast, some fish start reproducing as a female and later switch to being male, because large size is needed for males to defend resources and territories. Kazancioğlu and Alonzo 2010 illustrates that most empirical investigations have supported predictions of optimal switching points, but there can be many variables that modify the timing of sex change. Barrett, S. C. H., and J. Hough. 2013. Sexual dimorphism in flowering plants. Journal of Experimental Botany 64.1: 67–82. Reviews the ecological consequences of sexual dimorphism in dioecious plants, and the genetic and evolutionary processes that give rise to dimorphism. Available online for purchase or by subscription. Barrett, S. C. H., S. B. Yakimowski, D. L. Field, and M. Pickup. 2010. Ecological genetics of sex ratios in plant populations.Philosophical Transactions of the Royal Society B 365.1552: 2549–2557. Summarizes ecological and genetic mechanisms responsible for sex-ratio variation in plants. Although flower polymorphisms are maintained by frequency-dependent selection (as discussed in Fisher 1930, cited under Sex Ratios and Sex Allocation), the authors suggest that variation in other life history traits and demographic factors can cause deviations from a 1:1 sex ratio in dioecious plants. Available online for purchase or by subscription. Charlesworth, D. 1999. Theories on the evolution of dioecy. In Gender and sexual dimorphism in flowering plants. Edited by M. A. Geber, T. E. Dawson, and L. F. Delph, 33–60. Berlin: Springer-Verlag. Reviews of the origin and maintenance of dioecy. Freeman, D. C., H. T. Harper, and E. L. Charnov. 1980. Sex change in plants: Old and new observations and new hypotheses.Oecologia 47.2: 222–232. Reviews evidence for sex change in dioecious plants, and provides a simple model demonstrating that environmental heterogeneity favors the evolution of the ability to change sex, with females generally favored in high-quality environments and males favored in poor-quality environments. Available online for purchase or by subscription. Ghiselin, M. T. 1969. The evolution of hermaphoditism among animals. Quarterly Review of Biology 44.2: 189–208. An early attempt to identify conditions favoring hermaphroditism. Argues that most examples can be explained by one of three models: the low-density model (where mate-finding is difficult), the size advantage model (which favors sequential hermaphroditism), and the gene dispersal model (which addresses inbreeding and genetic drift in small populations). Availableonline for purchase or by subscription. Jacobs, M. S., and M. J. Wade. 2003. A synthetic review of the theory of gynodioecy. American Naturalist 161.6: 837–851. A critical review and novel synthesis of the theory underlying the evolution of, and maintenance of, gynodioecy. Available online for purchase or by subscription. Kazancioğlu, E., and S. H. Alonzo. 2010. Classic predictions about sex change do not hold under all types of size advantage.Journal of Evolutionary Biology 23.11: 2432–2441. Refines the size-advantage model for sequential hermaphroditism by considering different processes that can lead to a size advantage. The authors identify conditions in which a size advantage does not favor changing sex (e.g., when the male size advantage is a threshold function rather than a gradually increasing one). Available online for purchase or by subscription. Pannell, J. R. 2002. The evolution and maintenance of androdioecy. Annual Review of Ecology and Systematics 33:397–425. Reviews theoretical and empirical studies of androdioecy (populations consisting of males and hermaphrodites), which is much less common than dioecy or gynodioecy but has been identified in both plants and animals. The authors suggest that androdioecy typically evolves from dioecy, and provides reproductive assurance in small, colonizing populations. Available online for purchase or by subscription. Warner, R. R. 1975. The adaptive significance of sequential hermaphroditism in animals. American Naturalist 109.965: 61–82. An influential theoretical consideration of the size-advantage model of sequential hermaphroditism (following Ghiselin 1969, cited above). The models incorporate schedules of both age-specific mortality and age-specific reproduction to determine when switching sex is advantageous relative to remaining one sex throughout an individual’s lifetime. Available online for purchase or by subscription. Sex-Ratio Manipulation by Microorganisms Symbiotic and parasitic organisms commonly manipulate the sex ratio of their hosts (i.e., they are sex ratio distorters). For example, the rickettsia bacterium Wolbachia is an endosymbiont that lives inside insect cells. In addition to inducing parthenogenesis and causing cytoplasmic incompatibility, it can manipulate the sex of its host (turn males into females) and selectively kill males, all of which bias the population sex ratio toward females. Engelstädter and Hurst 2009 discusses how this manipulation of host reproduction by microbes is adaptive for the bacterium because it is transmitted strictly through females. Hornett, et al. 2009 provides an intriguing example of how endosymbiotic bacteria can lead to rapid sex ratio evolution and substantial spatial variation in sex ratios. Engelstädter, J., and G. D. D. Hurst. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annual Review of Ecology, Evolution, and Systematics 40:127–149. A broad review of the wide diversity of ways that microbes can manipulate host reproduction. Includes discussion of many mechanisms by which symbionts and parasites can manipulate population sex ratio, such as feminization or killing of males. Available online for purchase or by subscription. Hornett, E. A., S. Charlat, N. Wedell, C. D. Jiggins, and G. D. D. Hurst. 2009. Rapidly shifting sex ratio across a species range.Current Biology 19.19: 1628–1631. An empirical study comparing museum and contemporary populations of a tropical butterfly to determine the effect of male-killingWolbachia on spatial and temporal variation in butterfly sex ratio. Finds that the ecological and evolutionary dynamics of this butterfly-Wolbachia interaction can vary substantially over short periods of time. Available online for purchase or by subscription. Aging and Senescence Virtually all organisms exhibit senescence, an increase in the probability of death and a decline in fertility with increasing age. This observation is initially puzzling—senescence is clearly maladaptive, so why doesn’t selection eliminate it from natural populations? Not surprisingly, resolution of this question involves genetic constraints and fitness trade-offs, in this case with respect to the effects of gene expression early versus late in life, or with respect to the trade-off between reproduction and somatic maintenance. In this section, we first consider “intrinsic” or genetic explanations for patterns and rates of senescence. We then turn to the potential importance of extrinsic or ecological sources of mortality. These factors can influence rates of aging because selection on late-acting harmful alleles will be weak if there is high extrinsic mortality in earlier stages. As discussed below, variation in the typical pattern of senescence can be strongly linked to variation in other life history traits. WHY ORGANISMS SENESCE Early arguments for why organisms senesce were often circular or inconsistent with genetic and evolutionary principles; Kirkwood and Cremer 1982 reviews these initial explanations. The modern understanding of senescence traces back to a series of papers by Peter Medawar, beginning in 1946. Medawar 1952 noted that the magnitude of natural selection against a deleterious allele will vary with the age of the organism. Specifically, deleterious (harmful) mutations that act early in life will be quickly removed from a population. In contrast, selection against mutations that are harmful later in life, after the organism has reproduced and thus passed the mutation to some descendants, will be relatively weak. Consequently, later-acting deleterious mutations are less likely to be eliminated. The mutationaccumulation hypothesis predicts that the equilibrium frequency of a deleterious allele, which depends on the balance between mutation introducing the allele and selection removing it, will be higher for late-acting alleles. Williams 1957extended this idea by noting that a mutation that has negative effects late in life can actually be favored by selection if it simultaneously has positive effects on fitness early in life. For example, a mutation that reduces life span may nevertheless increase in frequency if it increases early survival or reproduction. In this case, there can be an antagonistic pleiotropy with respect to fitness, since the same allele has opposite effects on fitness at different stages of the life cycle. Hamilton 1966 developed the mathematical foundation of this idea, and Charlesworth 2000 reviews the historical development of this theory. Both the antagonistic pleiotropy and mutation accumulation hypotheses have been very influential and widely tested in evolutionary biology. In one of the first attempts to distinguish between these hypotheses, Rose 1984 imposed artificial selection on the age at reproduction and tested its effects on rates of senescence. Hughes and Reynolds 2005 reviews some of the many empirical tests that followed. An alternative and more mechanistic explanation for the evolution of senescence, reviewed by Kirkwood and Holliday 1979, is the disposable soma model. This model, which postulates that the trade-off of most importance is between energy allocated to reproduction versus somatic maintenance; for example, energy devoted to reproduction reduces the competency of molecular proofreading in dividing somatic cells, which inevitably leads to a degeneration of somatic tissue. Recent work, such as Greer, et al. 2011, has made great strides toward understanding the genetic (or genomic) and physiological mechanisms underlying mortality rates and senescence. Charlesworth, B. 2000. Fisher, Medawar, Hamilton and the evolution of aging. Genetics 156:927–931. A concise review examining the history of the evolutionary theory of senescence, from Fisher to the modern population-genetic perspective. Greer, E. L., T. J. Maures, D. Ucar, et al. 2011. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans.Nature 479 (17 November): 365–371. Experimental study demonstrating epigenetic inheritance of variation in lifespan of C. elegans. Available online for purchase or by subscription. Hamilton, W. D. 1966. The moulding of senescence by natural selection. Journal of Theoretical Biology 12.1: 12–45. A thorough theoretical exploration of many of the ideas proposed in Williams 1957, including an analysis of the antagonistic pleiotropy hypothesis and how patterns of extrinsic mortality affect rates of aging. Available online for purchase or by subscription. Hughes, K. A., and R. M. Reynolds. 2005. Evolutionary and mechanistic theories of aging. Annual Review of Entomology 50:421–425. Reviews the evolutionary theory of senescence and experimental tests of the mutation accumulation and antagonistic pleiotropy models. Concludes that antagonistic pleiotropy is a frequent cause of senescence, but that the significance of mutation accumulation is less clear. The paper also reviews a diversity of proximate mechanisms that cause of aging. Available online for purchase or by subscription. Kirkwood, T. B. L., and T. Cremer. 1982. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Human Genetics 60.2: 101–121. A review of hypotheses proposed to explain senescence, starting with those of Weismann and progressing through modern ideas. Kirkwood and colleagues are proponents of the disposable soma theory of aging, so much of the review addresses this hypothesis. Available online for purchase or by subscription. Kirkwood, T. B. L., and R. Holliday. 1979. The evolution of ageing and longevity. Proceedings of the Royal Society B 205.1161: 531–546. A detailed presentation of the disposable soma theory. The paper reviews and critiques the mutation accumulation and antagonistic pleiotropy hypotheses, then expands on the disposable soma hypothesis originally postulated earlier. Available online for purchase or by subscription. Medawar, P. B. 1952. An unsolved problem of biology. London: H. K. Lewis. One of a series of influential papers by Medawar proposing that the magnitude of natural selection against a deleterious allele will be relatively weak late in life, after an organism has started to reproduce. Medawar’s ideas became the foundation for much of our understanding of the evolution of senescence. Rose, M. R. 1984. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38.5: 1004–1010. Summarizes and replicates a series of previous tests of senescence theory. The authors artificially selected for high, late fecundity in females and found that early fecundity decreased and life span increased. Papers by Rose and colleagues prompted a vigorous discussion in the literature. Available online for purchase or by subscription. Williams, G. C. 1957 Pleiotropy, natural selection, and the evolution of senescence. Evolution 11.4: 398–411. The most influential early treatment of the evolution of senescence, proposing the antagonistic pleiotropy hypothesis. Williams describes the expected consequence of high versus low extrinsic mortality rates (including sex differences caused by sexual selection), proposes the first adaptive hypothesis for the evolution of menopause (described below), and discusses senescence in clonal organisms. Available online for purchase or by subscription. EXTRINSIC MORTALITY AND SENESCENCE IN AN ECOLOGICAL CONTEXT Williams 1957 (cited under Why Organisms Senesce) showed early on that extrinsic mortality factors (such as predation, disease, or starvation) can affect rates of aging. High extrinsic mortality, which is often age- and condition- independent, should cause late-acting deleterious mutations to accumulate faster than they would in populations experiencing low extrinsic mortality rates. If high extrinsic mortality causes fewer individuals to survive to old age, selection on late-acting alleles is reduced further, and early reproduction becomes even more beneficial. Theoretical models, like that of Abrams 1993, generally support this prediction, as does some experimental work, including the fruit-fly study of Stearns, et al. 2000. However, effects of extrinsic mortality on senescence can be complex. A longterm, experimental-evolution study, Reznick, et al. 2004, found that selection on life span and aging can be simultaneously affected by several variables in natural populations. Additional studies in natural populations have concluded that, although senescence is nearly universal, it can be hard to identify and quantify. Monaghan, et al. 2008 notes potential pitfalls in estimating rates of senescence, and Nussey, et al. 2013 demonstrates how the rate of senescence can vary substantially among traits, among individuals, and among environments. Each of these factors therefore needs to be incorporated into theoretical models and empirical studies of aging. Abrams, P. A. 1993. Does increased mortality favor the evolution of more rapid senescence? Evolution 47.3: 877–887. Theoretical exploration of how the extrinsic rate of mortality affects the evolution of senescence. Confirms prediction in Williams 1957(cited under Why Organisms Senesce) that that greater extrinsic mortality favors more rapid senescence, but also that higher extrinsic mortality can affect senescence in other ways, depending on specific ecological conditions. Available online for purchase or by subscription. Monaghan, P., A. Charmentier, D. H. Nussey, and R. E. Ricklefs. 2008. The evolutionary ecology of senescence. Functional Ecology 22.3: 371–378. Argues that there are novel insights to be gained by estimating senescence in nature, and calls for more research into senescence of traits other than survival (such as fecundity), the use of alternative methods to measure senescence (other than simple age at death), and better recognition of environmental sources of variation in senescence. Available online for purchase or by subscription. Nussey, D. H., H. Froy, J.-F. Lemaitre, J.-M. Gaillard, and S. N. Austad. 2013. Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Research Reviews 12.1: 214–225. A brief overview of evolutionary senescence theory, debunking the fallacy that senescence should be uncommon in wild populations, and reviewing sources of variation in senescence in wild populations. This paper is particularly valuable because most tests of senescence theory have been performed in the laboratory, often with model species. Available online for purchase or by subscription. Reznick, D. N., M. J. Bryant, D. Roff, C. K. Ghalambor, and D. E. Ghalambor. 2004. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 431 (28 October):1095–1099. A test of the hypothesis that extrinsic mortality rates affect the evolution of age-specific mortality rates. The effect on senescence was trait-specific, but the results were generally inconsistent with the prediction that senescence should increase when extrinsic mortality risk is high. See also “Testing Evolutionary Theories of Aging in Wild Populations,” by A. Bronikowski and D. E. L. Promislow, inTrends in Ecology and Evolution 20.6 (2005): 271–273. Available online for purchase or by subscription. Stearns, S. C., M. Ackermann, M. Doebeli, and M. Kaiser. 2000. Experimental evolution of aging, growth, and reproduction in fruitflies. Proceedings of the National Academy of Sciences 97.7: 3309–3313. Illustrates that the evolutionary changes in senescence are typically correlated with changes in other life history traits. Experimental evolution using Drosophila confirmed that imposing higher extrinsic mortality rates (and reduced selection against late-acting deleterious alleles) caused the predicted increase in intrinsic mortality rates (senescence) and shorter life spans. Available online for purchase or by subscription. MENOPAUSE In some organisms, females survive for a moderate or substantial amount of time even after they become postreproductive. Post-reproductive life spans have received extensive attention in humans, in which females undergo menopause—the relatively abrupt loss of ovarian function, with a spectrum of physiological consequences. In contrast, male fertility in humans declines gradually. Why females undergo menopause, and often have a long postreproductive life span, has been a topic of substantial debate. One hypothesis is that the post-reproductive life span is an artifact of recent, unnaturally long life spans in humans. However, as Cohen 2004 shows, this phenomenon is also observed in a variety of other animals in nature. There are also multiple adaptive hypotheses to explain menopause, including the mother hypothesis proposed in Williams 1957 (cited under Why Organisms Senesce) and thegrandmother hypothesis discussed in Hawkes, et al. 1998. Both of these hypotheses postulate that menopause improves female fitness through indirect effects on offspring fitness. Under standard kin-selection models, females in species with a long period of juvenile dependency may improve their inclusive fitness by refraining from having more offspring and instead directing care to their remaining offspring or even to grandoffspring. An alternative explanation is the reproductive competition hypothesis, proposed in Cant and Johnstone 2008. Though these competing ideas have mostly been tested using human datasets, a few tests have been performed in natural populations. These studies include Packer, et al. 1998 and Ward, et al. 2009. Cant, M. A., and R. A. Johnstone. 2008. Reproductive conflict and the separation of reproductive generations in humans.Proceedings of the National Academy of Sciences 105.14: 5332–5336. Presents the reproductive competition hypothesis for the evolution of menopause. The authors argue that the relatedness asymmetry that results from female-biased dispersal favors younger females in reproductive competition with older females, and thus selects for older females to cease reproducing. Available online for purchase or by subscription. Cohen, A. A. 2004. Female post-reproductive lifespan: A general mammalian trait. Biological Reviews 79.4: 733–750. An extensive review of female reproductive cessation in mammals, concluding that most species undergo some degree of reproductive cessation at older ages. Also provides an overview of adaptive and nonadaptive hypotheses proposed before 2004 to explain menopause. Available online for purchase or by subscription. Hawkes, K., J. F. O’Connell, N. G. Blurton-Jones, H. Alvarez, and E. L. Charnov. 1998. Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of Sciences 95.3: 1336–1339. An early development of the grandmother hypothesis, which was originally presented in the human biology literature. Available onlinefor purchase or by subscription. Packer, C., M. Tatar, and A. Collins. 1998. Reproductive cessation in female mammals. Nature 392 (23 April): 807–811. A demonstration that a reproductive decline analogous to menopause occurs in olive baboons and African lions, species that do not appear to conform to the grandmother hypothesis. Elderly females did not suffer increased mortality if they reproduced, and post-reproductive females did not increase the fitness of their children or grandchildren. Available online for purchase or by subscription. Ward, E. J., K. Parsons, E. E. Holmes, K. C. Balcomb III, and J. K. B. Ford. 2009. The role of menopause and reproductive senescence in a long-lived social mammal. Frontiers in Zoology 6:4. Experimental test of the grandmother hypothesis in killer whales. The results provided mixed support; grandoffspring did not have higher overall survival when grandmothers were present, but there was some evidence that grandmothers positively affected survival of juveniles at a critical life stage. SEXUAL SELECTION AND SENESCENCE A common observation in animals is that males have shorter adult life spans than do females, which leads to the oftstated idea that natural selection has acted to produce a “live fast, die young” type of life history for males. Why males typically senesce more quickly, independent of current reproduction, has been challenging to explain. Recent research has invoked a role of sexual selection and, in particular, sexual conflict to explain sex differences in mortality rates. Promislow 2003 reviews the hypothesis that, because of sexual selection, male reproductive strategies will typically be associated with elevated mortality risks and weaker selection for long life span, relative to female reproductive strategies. Differences in sexual selection between males and females can affect aging in a couple of ways, as described by Bonduriansky, et al. 2008. The greater variance in reproductive success among individual males (compared to females, due to sex differences in reproductive investment) creates a situation in which males can gain significant mating success by allocating resources to mating at the expense of longevity, whereas females gain little by doing so. Active foraging for females and engaging in direct male-male competition can lead greater physical costs and higher rates of extrinsic mortality that can, in turn, lead to the evolution of more rapid aging. Tests of these sexual selection hypotheses for aging are few, and results thus far have generally been inconsistent. An early study, Fowler and Partridge 1989, showed that mating can have direct effects on survival, and male mating strategies can impose selection on female longevity. Johnstone and Keller 2000 lists ways that males can benefit by causing physical damage to females and by manipulating female reproduction (e.g., through substances in the ejaculate). The degree to which harming or manipulating females is beneficial to males varies with the mating system; experiments with fruit flies (Holland and Rice 1999) suggest that natural selection favors greater reproductive cooperation in more monogamous species, which in turn directly affects the evolution of senescence. Any theoretical consideration of the relationship between sexual selection and aging must also explain circumstances under which increased male longevity is expected (e.g., when older males have higher mating success than younger males, or when sex roles are “reversed” and females show high variance in mating success). Finally, it is worth noting that the arrow of causation can be reversed; that is, existing rates of senescence and aging in a population can influence the form and strength of sexual selection. Bonduriansky, R., A. Maklakov, F. Zajitschek, and R. Brooks. 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Functional Ecology 22.3: 443–453. Review of the conceptual ideas and the empirical evidence linking sexual selection and rates of aging. Concludes that there are many testable hypotheses regarding the link between sexual selection and aging, but that empirical studies are currently too few to adequately test most of these hypotheses. Available online for purchase or by subscription. Fowler, K., and L. Partridge. 1989. A cost of mating in female fruitflies. Nature 338 (27 April):760–761. Previous studies had demonstrated that exposure to males and increased mating rate reduce female longevity. This study attempted to disentangle the direct effects of mating on female aging from the indirect effects of mating (via increased reproduction). The authors conclude that mating is costly to females independent of its effect on nutrient allocation to reproduction. Available online for purchase or by subscription. Holland, B., and W. R. Rice. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proceedings of the National Academy of Sciences 96.9: 5083– 5088. Experimentally manipulated sexual conflict in Drosophila melanogaster by maintaining flies under monogamous versus promiscuous mating conditions. Males selected under monogamy evolved to be less harmful to their mates, and monogamous females had higher reproductive rates, suggesting a substantial role of sexual conflict in the evolution of fly life histories. Availableonline for purchase or by subscription. Johnstone, R. A., and L. Keller. 2000. How males can gain by harming their mates: Sexual conflict, seminal toxins, and the cost of mating. American Naturalist 156.4: 368–377. Previous studies had demonstrated that males can cause harm to females during mating, reducing female reproduction and survival. This paper explores the hypothesis that imposing mating costs—including physical damage and toxins in the seminal fluids—is adaptive for males. Available online for purchase or by subscription. Promislow, D. 2003. Mate choice, sexual conflict, and evolution of senescence. Behavior Genetics 33.2: 191– 201. Though multiple previous papers had noted the potential role of sexual conflict in the evolution of senescence, in this article Promislow clarified the conceptual issues and provided guidance for future research on the role of sexual selection and sexual conflict in the evolution of senescence. Available online for purchase or by subscription. SENESCENCE IN MODULAR AND CLONAL ORGANISMS Many organisms, including most plants and many animals, are modular; they consist of repeating, similar units. The degree of integration among modules varies among organisms. At one extreme they may exhibit substantial integration, so that any physiological changes are necessarily shared among units. At the other extreme, each module is effectively a separate individual, even if all modules were produced clonally and are genetically identical. Age-specific patterns of reproduction and survival can vary considerably among modules. Although modular organisms are subject to the same evolutionary processes as all other organisms, traditional aging is complicated by this variation in the interdependence of modules, and separation of senescence of the whole “organism” from the senescence of individual modules can be difficult. For example, adaptive remobilization of resources among modules (e.g., resource transfer from nonreproductive modules to reproductive ones) can occur throughout the life cycle of the plant, and can lead to senescence and death of particular modules independent of the rate of aging of the whole organism. Also, some modular organisms, and many organisms that are not modular, reproduce clonally, such that the germ and somatic cell lines are not separate. The complexity of aging in clonal and multicellular organisms is too great to review here. Recent discussions of senescence in perennial plants are found in Munné-Bosch 2008 and Peñuelas and Munné-Bosch 2010. Aging in clonal animals is discussed in Sköld and Obst 2011, and aging in bacteria is reviewed in Książek 2010. Książek, K. 2010. Bacterial aging: From mechanistic basis to evolutionary perspective. Cellular and Molecular Life Sciences67.18: 3131–3137. Reviews the mechanistic basis and evolutionary significance of aging in bacteria. In particular, the paper discusses the significance of asymmetric cell division and the stationary phase of population growth. Available online for purchase or by subscription. Munné-Bosch, S. 2008. Do perennials really senesce? Trends in Plant Science 13.5: 216–220. A review of senescence in perennials that contrasts senescence of cells/tissues/organs with that of meristems and the whole plant. Available online for purchase or by subscription. Peñuelas, J., and S. Munné-Bosch. 2010. Potentially immortal? New Phytologist 187.3: 564–567. A brief commentary arguing that modular growth, developmental plasticity (and thus the ability to regenerate organs), and meristem dormancy make the likelihood of dying from aging insignificant relative to external sources of mortality in many perennial plants. Available online for purchase or by subscription. Sköld, H. N., and M. Obst. 2011. Potential for clonal animals in longevity and ageing studies. Biogerontology 12.5: 387–396. Discussion of senescence in clonal animals. Reviews the evidence for individual- versus colony-level senescence in metazoans, and the mechanisms by which clonal animals delay senescence (e.g., up-regulation of telomerase and regeneration. Availableonline for purchase or by subscription. LAST MODIFIED: 09/30/2013 DOI: 10.1093/OBO/9780199830060-0016 BACK TO TOP Oxford University Press