Mystery Liquids - The Discovery Museums
advertisement

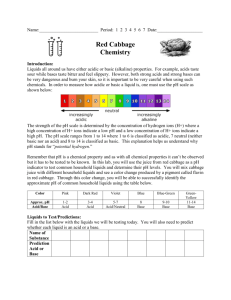
Reaction Station: Adventures in Chemistry Red Cabbage Juice Natural Acid/Base pH Indicator Supplies 2- 3 Reaction Boxes Signs Gloves Goggles Mystery liquids – acid/base Red Cabbage, fresh or frozen Blender Bags to dispose cabbage pulp 2 white mats for bottom of Reaction Station box White tray for observing Cutting board Knife Colander Pan Funnel 1,000 ml Erlenmeyer Flask for strained Cabbage Juice Bottle of rinse water Container for used liquid and rinse water Test tube drying racks Labeled containers of: o Vinegar o Sprite o Baking Soda o Diluted Ammonia - Windex For each box: 250 or 500 ml Erlenmeyer flasks for Cabbage juice Long pipette in Cabbage Juice Numbered (1-4) square containers in tray with: o Vinegar o Sprite o Baking Soda o Diluted Ammonia Short droppers in each liquid Reaction Station: Adventures for Young Chemists funded by the Camille & Henry Dreyfus Foundation Contact: dleblanc@discoverymuseums.org Test tube tray 5 test tubes for each set 6-well clear plastic egg-cartons for each set in white tray Additional materials: Ph paper – litmus strips Goldenrod paper (base indicator) Test tube tongs Additional Mystery Liquids: o Lemon or lime juice, milk, salad dressing, ketchup, soap, baking powder, etc. Make Cabbage Juice: Cut ½ of red cabbage into 2” pieces and place in blender. Add water to fill blender. Cover and blend until cabbage is pulverized. Pour slurry through a strainer and collect filtered juice in a pan or bowl. Use the filtered cabbage indicator. The pulp will also change colors with acids and bases. Be sure to discard the pulp in sealed plastic bags! Set up Reaction Stations with test tubes or clear plastic egg-carton wells: Reaction Station: Adventures for Young Chemists funded by the Camille & Henry Dreyfus Foundation Contact: dleblanc@discoverymuseums.org Natural Acid/Base Indicator – Red Cabbage Juice Do the students or museum visitors know about Acids/Bases, pH scale, litmus? Do they know the characteristics? If they don’t, or are young , they can make observations and see color changes. Mystery Liquid testing: 1. Place one pipette or dropper full of liquid 1 in the first well or test tube. 2. Place on pipette full of liquid 2 in the second, etc. 3. Do you see any color? 4. Add five drops of the purple cabbage extract into each test tube or well. Do not dip the pipette into the clear liquids. 5. Put one pipette full of the cabbage extract into an empty test tube or well. This is the control – original color of indicator. 6. What do you notice? Extensions: Mix acid and base (vinegar and baking soda) to see reaction and color change Collect a sample of a mystery liquid at the Haz-Mat site. Test it in the “lab”. What does the color tell you about it? Test other liquids, acids and bases – hand soap, dishwashing soap, lemon juice, milk, Use grape juice as an indicator. How does it compare? Test with Goldenrod paper, or turmeric solution Titration/Explosion/Baking soda-Vinegar Challenge Mix acid and base (vinegar and baking soda) to see reaction and color change Find right amount of baking soda and vinegar to produce explosion 15 ml graduated test tubes o What is the smallest amount of vinegar you can use? o What is the least amount of baking soda you can use? o Is the reaction different if you add baking soda to vinegar or vinegar to baking soda? Goldenrod Paper – base indicator Use to narrow down 4 mystery liquids into acid and base – in field, litmus paper. Test with Goldenrod Paper, or turmeric solution o Goldenrod Paper (Teacher Source/Education Innovations) Try different bases: diluted soap, ammonia, baking soda solution, other cleaners Haz-Mat Team – environmental response Collect a sample of a mystery liquid at the Haz-Mat site. Test it in the “lab”. What does the color tell you about it? Test other liquids, acids and bases – hand soap, dishwashing soap, lemon juice, milk, Use grape juice as an indicator. How does it compare? Reaction Station: Adventures for Young Chemists funded by the Camille & Henry Dreyfus Foundation Contact: dleblanc@discoverymuseums.org Mystery Clear Liquid Observations Describe the properties 1. 2. 3. 4. Test with indicator – goldenrod paper 1. 2. 3. 4. Test mystery liquids with natural indicator in Reaction Station 1. 2. 3. 4. Reaction Station: Adventures for Young Chemists funded by the Camille & Henry Dreyfus Foundation Contact: dleblanc@discoverymuseums.org