Chapter 9 Answers
advertisement
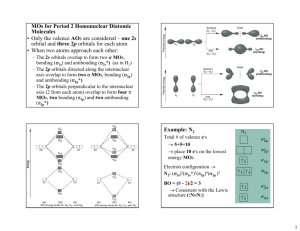
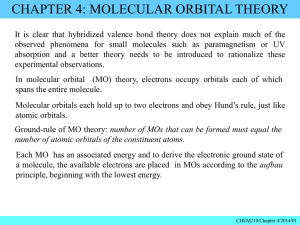
Written by: Katherine Lee Period 4 AP Chem Ms. Vorak AP CHEMISTRY CHAPTER 9—HYBRIDIZATION, THE LOCALIZED ELECTRON & MOLECULAR ORBITAL MODEL MULTIPLE CHOICE QUESTIONS ANSWERS 1.) The molecular orbital energy level organization of N2 is: a. σ1s, σ1s*, σ2s, σ2s*, σ2p, σ2p*, π2p, π2p* b. σ1s, σ1s*, σ2s, σ2s*, π2p, π2p*, σ2p, σ2p* c. σ1s, σ1s*, σ2s, σ2s*, σ2p, π2p, π2p*, σ2p* d. σ1s, σ1s*, σ2s, σ2s*, π2p, σ2p, π2p*, σ2p* e. σ1s*, σ1s, σ2s*, σ2s, π2p*, σ2p*, π2p, σ2p 2.) How many σ and π bonds does butene have? a. 10 σ and 1 π bonds b. 11 σ and 1 π bonds c. 11 σ and 0 π bonds d. 10 σ and 0 π bonds e. None of the above 3.) Calculate the bond order of He2 a. 0 b. 2 c. 4 d. ½ e. He2 doesn’t have a bond order 4.) The following molecule has how many atoms that are sp3 hybridized? b. c. d. e. f. 4 5 9 10 11 5.) The O2 molecule is which of the following? a. sp bonded b. Diamagnetic c. Paramagnetic d. Both a and c e. None of the above 7 Written by: Katherine Lee Period 4 AP Chem Ms. Vorak AP CHEMISTRY CHAPTER 9—HYBRIDIZATION, THE LOCALIZED ELECTRON & MOLECULAR ORBITAL MODEL FREE RESPONSE QUESTION ANSWERS A.) The following questions will be based on the following molecule, 11-cis retinal 1.) Please draw the complete structure of 11-cis retinal, complete with all atoms and lone pairs 2.) 11-cis retinal has how many sp2 and sp3 hybridized C atoms? 11 sp2 and 9 sp3 C atoms 3.) Explain the phenomenon of π bonding in the molecule, and identify the number of π bonds in 11-cis retinal Because some of the carbons in 11-cis retinal are sp2 hybridized, there is a pair of un-hybridized pz orbitals with unpaired electrons. Because electrons are considered delocalized, that is having no one set area of occurance, they form a bond, which is called π bonding, which allows for the extra strength of the double-bonded carbons. There are 11 π bonds in 11-cis retinal. B.) Please draw the last four energy levels of the Molecular Orbital Model for C2. σ2p* ↑↓ π2p* ↑↓ ↑↓ σ2p ↑↓ π2p ↑↓ ↑↓ 1.) Explain why its orbitals are reversed Due to s-p orbital mixing in the molecular orbitals, the energies of the π2p and σ2p orbitals are switched, making the π2p orbital the lowest in energy. Also, C2 is diamagnetic, and the MO diagram with the reversed π2p and σ2p orbitals accurately represent that, whereas the normal orbital organization would have labeled C2 as paramagnetic. 2.) Why can’t this molecule be picked up by a magnet? C2 is paramagnetic, because its orbitals all have paired electrons. Therefore, it is unattracted to a magnetic field. 8