Exploring the Periodic Table
advertisement

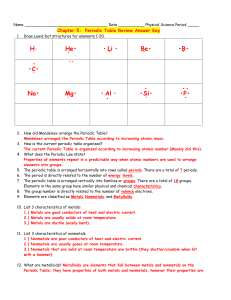
Name: ____________________ # _____ Date: _________________Core: _____ Exploring the Periodic Table With the development of the atomic theory came a great deal of knowledge about how and why the world appears as it does. There is no argument that atoms of elements are the building blocks of matter and understanding the atoms that make up these elements is very important to the study of matter and energy today. In an attempt to understand the properties of the elements and how to use the periodic table to your advantage, you will step into the role of a real chemist and try your hand at working with the periodic table. Fortunately, you will not have as difficult a time as Dmitri Mendeleev who produced the first periodic table in 1869. This lab is divided into 4 parts which will require you to move from station to station in the room, per your teacher’s directions. Activity 1: Using the Periodic Table Directions: Refer to the textbook (p. D20—30) to help you answer #1—9 below. 1) Tungsten is an element that is used to create the filaments in a light bulb. Find tungsten on the periodic table and record: Tungsten Symbol Atomic number Atomic mass How many protons? How many electrons? How many neutrons? 2) How many elements were known when this book was published? __________ 3) What determines the order in which the elements are arranged? 4) What do you see most often (at standard temperature and pressure): solid, liquid, gas? 5) Which 2 elements are liquids? 6) About what percent of the elements are: metals __________ metalloids __________ nonmetals __________ *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ 7) What do you notice about the location of the following (in general): metals-metalloids-nonmetals-8) Read pages D22-23 in your text and answer the following: a) What is each horizontal row known as on the periodic table? b) Do elements change in predictable ways from one end to the other in these rows? c) What is each vertical column known as on the periodic table? d) Are the elements in these vertical rows related? Explain. 9) Read p. D26. What is reactivity? How can you use the periodic table to determine how reactive an element might be? *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ Activity 2 — Metals, Nonmetals, and Metalloids Directions: You will be given different elements from the periodic table. You will be exploring some properties of metals, nonmetals, and metalloids. **Note: To test if it is malleable/brittle, use a nail to see if the element flattens (malleable) or breaks (brittle).** Element Color State (solid, Luster liquid, gas) Malleable/ Brittle Conducts electricity? Conducts heat? Metal, Nonmetal, or Metalloid Sulfur Magnesium Carbon Silicon Aluminum Summary 1) Summarize properties of metals. 2) Summarize properties of nonmetals. 3) Summarize properties of metalloids. 4) What state of matter occurs most frequently? 5) In examining the periodic table, what do you notice about elements that are not solids? *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ Activity 3—Discovering Relationships within the Groups Directions: You will be assigned an element from your teacher. Find the other students in the class who were assigned an element in your same group. Using the information from the periodic table and group characteristics identified in your textbook (p. D20—30) fill in the chart below with your group members. What element have you been assigned? _________________________________________ Step 1 Complete the table below. 1) List all the elements in the group from lowest atomic number to highest (include any elements that were not assigned to a student in your group). 2) Are the elements in the group classified as metals, metalloids, or nonmetals? 3) Do the elements in your group have any properties in common? If so, which? 4) What uses do the elements in your group have in common? 5) How many valence electrons are there in the atoms of this group? 6) How reactive are the elements in this group (very, reactive, mild, low or none)? Do the atoms tend to gain, lose or share electrons when chemically bonding? 7) Are there any trends in reactivity as you move from the lowest atomic number to the highest atomic number in your group? Step 2 Using the materials provided, create a visual to highlight the key features of your group. If your group has a distinct name, be sure to write it big enough to be seen from a short distance. The poster should also include the total number of metals, nonmetals and metalloids in the group, the group’s reactivity as a whole, the number of valence electrons, similar properties, uses, and the number of valence electrons lost or gained when bonding. *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ Activity 4: Comparing the Groups Group Name and Number Metal, Nonmetals, or Metalloid (number of each) Number of Valence Electrons # of Valence Electrons Gained/Lost (+/-) Reactivity (Very, reactive, mild, low or none) Alkali Metals (1) Alkaline Earth Metals (2) Boron Group (13) Carbon Group (14) Nitrogen Group (15) Oxygen Group (16) Halogens (17) Noble Gases (18) Two major trends regarding reactivity: Metals tend to ___________ valence electrons when combining with other atoms, and nonmetals tend to __________ valence electrons when combining with other atoms. The __________ valence electrons an atom has to gain OR lose when combining with other atoms, the __________ reactive it is. (*Exception: __________ __________) WORD BANK more lose gain less *adapted lab from FCPS Name: ____________________ # _____ Date: _________________Core: _____ Summary—Exploring the Periodic Table 1) In your own words, briefly summarize what you have learned about the major groups on the periodic table. 2) Differentiate between metals, nonmetals and metalloids…what are some unique characteristics of each? Application 1) In an engineering class, you and your lab partner are asked to build a working model for new blimp design. Your partner wants to use hydrogen gas to fill the blimp but you suggest helium gas would be a better option. Based on what you learned in this lab, why is helium gas the better choice? 2) Using the information from this lab regarding the organization of the periodic table, answer the following questions. For each, circle your choice and indicate the reasoning behind your answer. a) Which element is more reactive? lithium (Li) or phosphorus (P) Reasoning: bromine (Br) or germanium (Ge) Reasoning: b) Which element is considered a metalloid? aluminum (Al) or silicon (Si) Reasoning: c) Which element would be considered the best conductor of electricity? strontium (Sr) or iodine (I) Reasoning: d) Which element would be the most brittle (fragile/easily broken into pieces)? boron (B) or sulfur (S) Reasoning: *adapted lab from FCPS