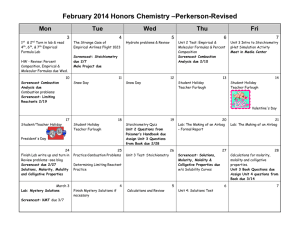
Unit 6 – Stoichiometry
advertisement
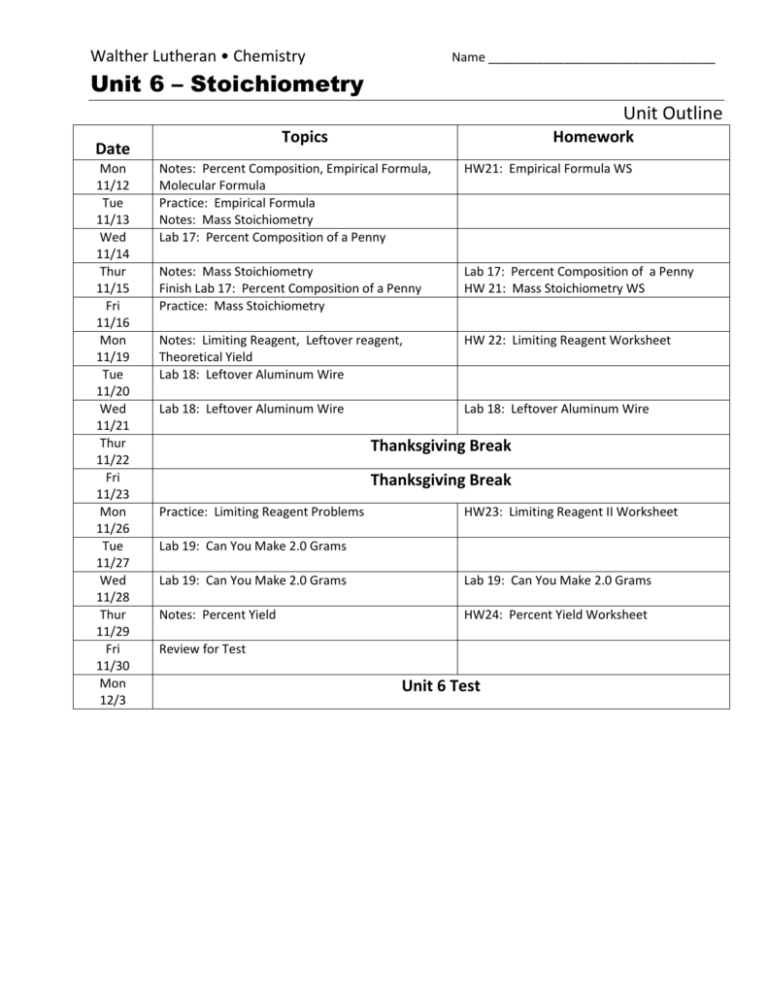
Walther Lutheran • Chemistry Name _________________________________ Unit 6 – Stoichiometry Unit Outline Topics Date Mon 11/12 Tue 11/13 Wed 11/14 Thur 11/15 Fri 11/16 Mon 11/19 Tue 11/20 Wed 11/21 Thur 11/22 Fri 11/23 Mon 11/26 Tue 11/27 Wed 11/28 Thur 11/29 Fri 11/30 Mon 12/3 Homework Notes: Percent Composition, Empirical Formula, Molecular Formula Practice: Empirical Formula Notes: Mass Stoichiometry Lab 17: Percent Composition of a Penny HW21: Empirical Formula WS Notes: Mass Stoichiometry Finish Lab 17: Percent Composition of a Penny Practice: Mass Stoichiometry Lab 17: Percent Composition of a Penny HW 21: Mass Stoichiometry WS Notes: Limiting Reagent, Leftover reagent, Theoretical Yield Lab 18: Leftover Aluminum Wire HW 22: Limiting Reagent Worksheet Lab 18: Leftover Aluminum Wire Lab 18: Leftover Aluminum Wire Thanksgiving Break Thanksgiving Break Practice: Limiting Reagent Problems HW23: Limiting Reagent II Worksheet Lab 19: Can You Make 2.0 Grams Lab 19: Can You Make 2.0 Grams Lab 19: Can You Make 2.0 Grams Notes: Percent Yield HW24: Percent Yield Worksheet Review for Test Unit 6 Test Walther Lutheran • Chemistry Name _________________________________ Unit 6 – Stoichiometry Unit Outline Objectives: 1. 2. 3. 4. 5. 6. 7. 8. 9. Understand and be able to apply to calculations the concept of % by mass or Understand the term empirical formula Be able to calculate empirical formula from data Understand the relationship between empirical formula and molecular formula and be able to perform simple conversions between the two Understand that balanced equations give useful information about reacting ratio's of moles Be able to use those reacting ratios to calculate moles and masses of reactants and products from given data Understand and be able to use the concept of limiting reactant Understand and be able to use the concept of percentage yield Recall that in this topic you are required to write chemical formula and correct, balanced equations