PHYS 3324 Experiment 2: Atomic Spectra
advertisement

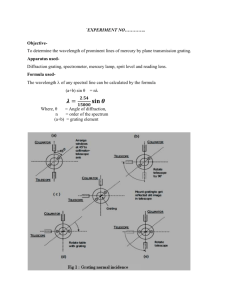
PHYS 3324 Experiment 2: Atomic Spectra Background Reading: Krane, pp. 185-189 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass, small flashlight. Prelab Questions: 1. What would be the diffraction angle, in first order, for a diffraction grating with a separation between rulings of 2 micrometers when observing the yellow sodium line? What would be the diffraction angle in second order? 2. Use the Rydberg-Ritz formula to calculate the wavelength for the n2 = 3 to n1 = 1 transition in hydrogen. Why can’t this line be observed with the diffraction grating? I. Introduction Atomic spectra will be studied in this experiment using a diffraction grating mounted in a spectrometer. An atomic transition in sodium will be used to calibrate the line spacing of the grating, then the grating will be used to determine the Rydberg constant in hydrogen. II. Spectrometer A schematic drawing of the spectrometer is shown in Fig. 1. Light from the source enters the collimator tube through an adjustable slit and is formed into a parallel beam by the collimator lens. The beam enters the diffraction grating which produces sharp interference maxima at angles that are characteristic of the wavelengths of the incident light. Light from the diffraction grating then passes into a telescope. The objective lens and eyepiece of the telescope form an image which the observer aligns with a crosshair to measure the diffraction angle. The spectrometer is calibrated by using the two bright lines of sodium (sodium D lines). These two lines, with wavelengths = 589.0 nm and 589.6 nm, are so closely spaced that they can be regarded as one line with a wavelength of 589.3 nm. 2-1 Figure 1. Spectrometer III. Diffraction Grating A diffraction grating is a sheet of celluloid which has a large number of evenly-spaced parallel lines ruled on it. The celluloid is sandwiched between glass plates. When parallel light is incident on such a grating, the light is diffracted with maxima at angles which satisfy the relation: d sin n (1) where d is the distance between successive rulings, is the wavelength of the incident light, and n is an integer called the order of the spectrum. This equation is derived in many elementary textbooks. Eq. 1 is not used directly in the experiment since it is difficult to align the diffraction grating exactly perpendicular to the light beam. It can be proved that, if the grating is misaligned, the diffraction maximum on one side of the beam, called n as in Fig. 2, is displaced nearly the same amount as the maximum on the other side, called n . Therefore, if half the difference between these angles is used in Eq. 1, misalignment errors 2-2 nearly cancel. Thus Eq. 1 becomes n d sin n n 2 (2) Figure 2. Diffracted beam from grating The spacing d is not known precisely for your grating. Therefore the spectrometer will be calibrated using a spectral line of known wavelength. Eq. 2 will be used in the form n sin n Kn 2 (3) where the constant K=1/d is determined in the calibration procedure. Note that it is necessary to measure a particular order of interference on both the left and right sides in order to use Eq. 3. Because most gratings do not produce equally intense light on both sides of the diffraction grating, some lines may be too weak to be seen on both sides in all orders. Such lines cannot be used. IV. Alignment The spectrometer has three components which must be aligned – the collimator, the telescope and the grating. See Fig. 3 for the relative position of these elements. 2-3 The collimator is used to produce parallel rays from a source of light placed by its slit. It consists of a main tube, fitted at one end with an adjustable slit, and at the other, a small draw tube containing a lens. The draw tube may be slid in or out of the main tube, so as to place the slit in the focal plane of the lens. Figure 3. Spectrometer The telescope is used to receive parallel rays of light from the collimator after they have been diffracted by the grating mounted on the optical stage. It consists of a main tube which is fitted at one end with an objective lens. The other end holds a draw tube fitted with an eyepiece containing focusable cross hairs. The draw tube may be slid in or out of the main tube so as to focus the telescope at infinity, and the eyepiece may be slid in or out of the draw tube to bring the crosshairs into focus. The telescope may be rotated about the central axis of the instrument and then clamped into position. There is a fine motion tangent screw for precise positioning of the telescope. The rotation of the telescope is controlled by using a locking screw below the telescope. This screw should always be unlocked when rotating the telescope. When unlocked, the telescope will move freely using only slight pressure. The parts need not be forced to 2-4 move. A tangent screw next to the locking screw is used for fine adjustments after the locking screw is tightened. The diffraction grating is supported by an optical stage which has an adjustable vertical position and can be rotated. The locking screw just below the grating should be loosened before the grating is rotated. The optical-stage locking screw at the base of the spectrometer (see Fig. 3) should be locked. It is not used in this experiment. The face of the diffraction grating is delicate and should not be touched. If you must handle the grating, hold it by the edges. Turn on the sodium lamp and let it warm up as you do the steps below. It needs about 10 minutes to get warmed up to stable operating conditions. The following steps will align the spectrometer: A. Telescope Rotate the eyepiece in the telescope clockwise and move in or out to obtain a sharp image of the crosshair. Rotate the eyepiece until one of the crosshairs is parallel to the plane of the rotation of the telescope. The other cross hair will then be vertical if the spectrometer is in a horizontal plane. If the spectrometer is not in a horizontal plane, see your lab instructor. Loosen lock #1 in Fig. 1 (just below the grating) and carefully lift and remove the grating platform. Rotate the telescope so that it points at a distant object such as a wall. With “Adj. A” shown in Fig. 1, focus the telescope so that the image of the wall coincides with the crosshairs. The position of the telescope objective lens (Adj. A) should not be changed during the rest of the experiment once this condition has been attained. However, the eyepiece can be readjusted if a different observer is to use the spectrometer. B. Collimator Subdue ambient lighting if necessary. Widen the slit on the collimator to about 0.5 millimeter by using knob “Adj. C” in Fig. 1. Swing the telescope out of the way and look through the collimator to observe the slit image of the source. Center the sodium source in front of the collimator to get the maximum intensity for the slit image. 2-5 Unlock the telescope, if necessary, and position it directly opposite the collimator so as to be able to view the image of the slit. Lock the telescope in place. Using metal knob “Adj. B”, slide the draw tube of the collimator in or out until the slit is seen in focus, with sharp edges and exhibiting no parallax in relation to the crosshairs. If the slit is not parallel to the vertical cross hair, loosen the locking ring at the slit and rotate the slit for parallel alignment with the crosshair. Then use the locking ring to clamp the slit in place. The collimator is now adjusted. C. Diffraction grating With knob #1 loosened, replace the optical stage with the grating aligned perpendicular to the collimator, as determined by eye. The mount should be rotated so that the grating is between its support and the telescope. Tighten knob #1. V. Apparatus Carry out the following preliminary operations to familiarize yourself with the spectrometer. Use a sodium lamp as the light source and set the telescope directly in line with the collimator so that you can see the image of the slit as a vertical yellow line. This is the zero order. Try adjusting the width of the slit by turning the small screw (Adj. C) at the outer end of the collimator. It is easier to work with a wider slit when looking for images and then narrow it down for accurate location and measurement. Observe that the telescope arm can be rotated around the grating table which is calibrated in degrees. A vernier scale moving with the telescope will allow readings to ± 1 minute. There is a clamping screw beneath the telescope and a fine adjustment screw at the side, which only operates when the telescope is locked. The fine adjustment screw allows the cross hair to be accurately located on the line to be measured. VI. Calibration of grating 1. Rotate the telescope so that it looks directly into the collimator at the image of the slit. Observe the central band (n = 0, zero order) slit image and reduce the slit width to form a thin bright image that can be easily observed. 2-6 2. Move the telescope arm to align the slit image with the vertical cross-hair. Lock the telescope in place with the locking screw, then use the tangent screw for fine adjustment of the crosshair. 3. Read the angular position of the telescope arm on the graduated scale; you will need the watchmaker eyeglass for this reading. Note that this reading is obtained from a vernier scale and can be measured to an accuracy of one minute of arc. Some sample vernier scale readings are presented in Fig. 4. Figure 4. Two examples of how to read the vernier scale Record the uncertainty with which the angular position can be measured. (Note that this uncertainty may be dominated by your ability to set the crosshair at the center of the finite width line, and not necessarily by your random reading error on the finest division of the vernier scale.) 4. Now place your eye to the right of the telescope and, looking into the diffraction grating, move back and forth to see the first order (n = 1) image of the slit. 5. Measure the first order diffraction angle by loosening the locking screw on the telescope and swing it to the right. An easy way to do this is to first swing your head over until you see the spectrum, then, without moving your head, swing the telescope in front of your eye. 2-7 6. Once again lock the telescope in place and use the tangent screw to align the crosshair with the center of the image. Read the angular position and record this measurement as the right side, first order (n = 1) angular position 1 . 7. Now place your eye to the left of the central (n = 0) position and look for the first order (n = 1) image. Measure this angle and record it as the left side, first order (n = 1) angular position 1 . 8. Use these angles and the wavelength of sodium (589.3 nm) to calculate K in Eq. 3 and, from K, the number of lines per cm on the diffraction grating. Check your result with the lab instructor before moving on. VII. Hydrogen Spectrum Turn off the sodium lamp and turn on the hydrogren lamp as you read the setup directions below; the lamp needs about 10 minutes to be fully warmed up. The visible lines in the hydrogen spectrum are shown on a wall chart in the laboratory. There are four: one red, one blue-green and two violet. The second violet line is considerably weaker than the first; it may not be visible. 1. Replace the sodium calibration source with the hydrogen lamp. Bring the lamp as close to the slit as possible. Be careful not to disturb the alignment of the spectrometer. 2. Adjust the position of the source to give maximum brightness for the zero-order maximum. This is most easily done by swinging the telescope out of the way. 3. Measure the position of the telescope for each of the visible lines in the hydrogen spectrum in first order. Once again, open the slit as you move between lines or between orders and close it for the final measurement. Identify each angle to indicate the corresponding color. Remember that it is crucial to observe both positive and negative orders of interference. VIII. Mercury Spectrum Remove the hydrogen source and replace it with the mercury (Hg) source. Give the Hg lamp about 10 minutes to warm up before making your measurements. Look at the wall chart and pick out three mercury lines to measure. Locate these lines and measure them for both positive and negative orders of interference (in first order). IX. Report Your lab report should address all of the following points. 2-8 Introduction Describe how you calibrated the diffraction grating. Include your measurements and show how you calculated the constant K in Eq. 3. Use your error estimate on the angle reading () to make an error estimate on K. Use your value of K to determine the numbers of rulings per cm of the diffraction grating you used. Determine the wavelengths of all the visible lines of hydrogen by means of Eq. 3 and your measured angles. Show a sample calculation of how you obtain the wavelength from your measured data. You should also use your error estimate on the angle reading () and on K (K) along with the propagation of error formulae to determine the error on each of your wavelength determinations. Show a sample calculation of this. Include everything (angle measurements, calculated wavelengths and their errors) in a table. The Rydberg-Ritz formula for the wavelengths in the spectrum of atomic hydrogen is 1 1 R 2 2 n1 n2 1 where n1 =2 for the visible Balmer series and n2 takes on positive integral values greater than 2. Using your measured values for , find by trial and error the proper values of n2 and then solve for the Rydberg constant R. Determine the error on each of your determinations of R (from the error on the wavelength). Determine your final value of R (and its error) by computing the weighted average of your determinations of R. Compare with the accepted value of R = 109678 cm-1. Finally, draw an energy level diagram for the hydrogen atom and indicate the transitions that correspond to each of the lines you observed. Determine the wavelengths (and errors) of the Hg lines you measured and compare them to the accepted values you read from the wall chart in class. Summarize these results in a table. Conclusion 2-9