Nucleophilic
advertisement

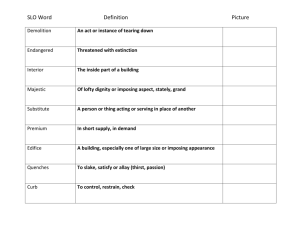
Experiment 8: Nucleophilic Substitution Reactions of Alkyl Halides Nucleophilic Substitution • Important reaction of alkyl halides • Two common mechanistic pathways: SN1: substitution, nucleophilic, unimolecular exp 1: Reaction with Sodium Iodide in Acetone exp 2: Reaction with Silver Nitrate in Ethanol RDS Reading: Carey & Guiliano Ch. 8 pgs 322-350 SN2: substitution, nucleophilic, bimolecular RDS Reaction Features Comparison: SN1 vs. SN2 • SN1 Reactions: - rate determining step involves ionizaton ! carbocation - reaction rate governed by cation stability - RX: 3° > 2° >> 1° • SN2 Reactions: SN1 intermediate carbocation no formal intermediate mechanism stepwise concerted kinetics 1st order 2nd order weak or strong strong polar, protic various cation stability sterics substrate 3° > 2° > 1° CH3X > 1° > 2° > 3° stereochemistry retention & inversion inversion rearrangements common not possible nucleophile solvent rate determined by - rate determining step involves backside attack of Nu - reaction rate governed by sterics - RX: 1° > 2° >> 3° SN2 Next Week Substrates (November 7 - 11) Experiment 8: Nucleophilic Substitution Reactions of R-X A. NaI in Acetone reaction via SN2 mechanism B. AgNO3 in ethanol reaction via SN1 mechanism NOTE: We will not do concentration studies for either part DUE: Alkyne Synthesis Lab Report (exp 7) Lab Reports are due at the beginning of your regular lab session Reactions Determine Characteristics of Nu Substitution • NaI in acetone (SN2) " - I- is a very good nucleophile - acetone is a polar, aprotic solvent - reaction will result in precipitate formation (NaCl or NaBr) • Evaluate the reactivity of the alkyl halide substrates considering: - primary structure (1°, 2°, 3°) - secondary structure (steric effects, proximity of double bonds) - nature of the leaving group (Br vs Cl) - concentration (substrate vs. reagent) - should understand - solvent (SN1 vs SN2 reaction) - reaction temperature (will evaluate indirectly) • AgNO3 in ethanol or ethanol/water (SN1) " - Ag+ in protic solvent - rapidly generates R+ and AgX (ppt) formation of ppt tells you reaction has occurred - subsequent reaction of R+ with solvent (ethanol), etc. • Qualitative evaluation - be ready to observe and record your results - watch for ppt formation - no products will be isolated - no yield will be determined Experimental Details - NaI in acetone (SN2) 1. Obtain microtubes & corks (8-10 each) - be sure tubes are clean & dry 2. Add 15% NaI/acetone (2 mL) to reaction tubes - more is not better 3. Add substrates to reaction tubes - 2 drops of substrate per reaction tube (~ 0.1 mL) - one substrate per tube - be sure to label them so you know what is in each one! - immediately stopper tubes, mix thoroughly, note start time - watch for ppt formation at room temperature record the exact time for ppt formation (if any) in each tube try to quantify how much ppt forms (a lot? just a little?) also record observations when no ppt forms Experimental Details - NaI in acetone (SN2) Specific Experiments: 1. Primary Structure - substrates: - expectations?: 1° > 2° >> 3° >>>> aryl 2. Secondary Structure - substrates: - expectations?: allylic > 1° unhindered > hindered 4. If no precipitate forms after ~ 5 minutes, heat to 50°C in hot water bath - note start time - watch for reaction (ppt formation) over next 4-5 minutes - record observations (e.g. reaction time; quantity of precipitate) Experimental Details - NaI in acetone (SN2) Specific Experiments (cont): 3. Leaving Group - substrates: - expectations?: Br > Cl (Br better able to accommodate negative charge) 4. Concentration (NOTE: we will not do this portion of the experiment) - substrate: - variations: change substrate concentration change Nu concentration - expectations?: both situations will result in an increase in reaction rate specifically, expect rate to double in each case because the reaction is bimolecular! k = [RX][I-] Experimental Details - AgNO3 in ethanol (SN1) 1. Empty microtubes from last set of reactions & wash with ethanol - DO NOT use acetone! 2. Add 1% AgNO3/ethanol (1 mL) to reaction tubes - more is not better 3. Add substrates to reaction tubes - 2 drops of substrate per reaction tube (~ 0.1 mL) - one substrate per tube (be sure you know what's in each one!) - immediately stopper tubes, mix thoroughly, note start time - watch for ppt formation at room temperature record the exact time for ppt formation (if any) in each tube try to quantify how much ppt forms (a lot? just a little?) also record observations when no ppt forms 4. If no precipitate forms after ~ 5 minutes, heat to 50°C in hot water bath - note start time - record observations as before 5. When finished, wash tubes (rinse well!), dry, & return - PLEASE do not put them in your drawer! Experimental Details - AgNO3 in ethanol (SN1) Experimental Details - AgNO3 in ethanol (SN1) Specific Experiments: Specific Experiments (cont): 1. Primary Structure - substrates: 3. Leaving Group - substrates: - expectations?: 3° > 2° >> 1° >>>> aryl - expectations?: Br > Cl (Br better able to accommodate negative charge) 2. Secondary Structure - substrates: - expectations?: allylic > 1° 4. Concentration (NOTE: we will not do this portion of the experiment) - substrate: why?? - variations: double substrate concentration double Nu concentration - expectations?: double [RX], expect reaction rate to double double [Nu], expect no change because the reaction is unimolecular! k = [RX] Experimental Details - AgNO3 in ethanol (SN1) Specific Experiments (cont): 5. Solvent - substrate: Writing the Lab Report: Exp #8 Alkyne Synthesis ! Purpose - what will you make? - include a balanced chemical equation for every reaction you did ! Results & Discussion - Comment on the success of your reaction sequence - variations: ethanol ethanol/water - expectations?: rxn in ethanol/water will be faster both are polar, protic solvents (favor SN1) pure water has a higher dielectric constant = more polar (really favors SN1) Did each step work? How do you know? ! physical appearance, TLC & melting point don’t forget to discuss the changes in Rf values that you observe - Were the products pure? How do you know? ! TLC & mp data Was your recrystallization successful for step 2? - Was the reaction sequence efficient? Calculate the % yield for each step Calculate the overall yield Writing the Lab Report: Exp #8 Alkyne Synthesis ! Conclusion - a brief recap of your findings - was your reaction sequence successful (did you make it? was it pure?) - do not include theory ! Appendix A: Calculations - Table of reagents (for each step) Calculating Overall Yield: • Reaction Sequence: 1. Calculate yield for each step (as discussed last week) - Determine the limiting reagent (for each step) - Percent Yield calculation (for each step) for step 2, keep in mind you may not have recrystallized everything see page 91 of the lab manual for details - Overall Yield calculation example follows - Rf calculation ! Appendix B: Spectra - none 87% 94% 2. Multiply individual yields together Overall Yield = (yieldstep 1) x (yieldstep 2)... x 100 = (0.87) x (0.94) x 100 = 82%