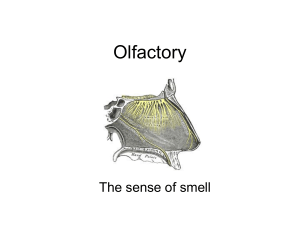
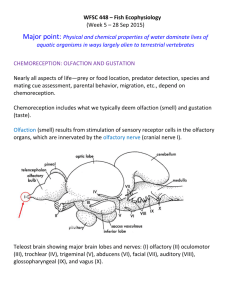
A Compartmental Model of a Spiking and Adapting Olfactory
advertisement

A Compartmental Model of a Spiking and Adapting Olfactory Receptor Neuron for Use in Large-Scale Neuronal Network Models of the Olfactory System CLÀUDIA RAMOS GARCIA Master of Science Thesis Stockholm, Sweden 2010 A Compartmental Model of a Spiking and Adapting Olfactory Receptor Neuron for Use in Large-Scale Neuronal Network Models of the Olfactory System CLÀUDIA RAMOS GARCIA Master’s Thesis in Biomedical Engineering (30 ECTS credits) at the IT Program Royal Institute of Technology year 2010 Supervisor at CSC was Malin Sandström Examiner was Anders Lansner TRITA-CSC-E 2010:050 ISRN-KTH/CSC/E--10/050--SE ISSN-1653-5715 Royal Institute of Technology School of Computer Science and Communication KTH CSC SE-100 44 Stockholm, Sweden URL: www.kth.se/csc A compartmental model of an olfactory receptor neuron “So take a deep breath, tell me what you smell. I smell a good time in my life... I think I like this city.” City. Billie the vision and the dancers A compartmental model of an olfactory receptor neuron A compartmental model of a spiking and adapting olfactory receptor neuron for use in large-scale neuronal network models of the olfactory system Abstract In the aim of obtaining complete models of the biological olfactory system it is necessary to develop models of each one of its parts: the olfactory epithelium (with olfactory receptor neurons), the olfactory bulb and the olfactory cortex. This thesis deals with the development of a model of the olfactory epithelium suitable for network simulations; in the form of a set of olfactory receptor neurons (ORNs) expressing different kind and amount of olfactory receptors. The developed ORN model is a single cell model at a very low level of abstraction, including Hodgkin-Huxley type ion channels. Taking experimental data as a reference, in this model I try to represent a good compromise between simplicity and biological plausibility. En spikande och adapterande kompartmentmodell av en luktreceptorcell för användning i storskaliga neuronala nätverksmodeller av luktsystemet Sammanfattning För att få en komplett modell av det biologiska luktsystemet är det nödvändigt att utveckla modeller för alla dess delar: luktepitelet (med luktreceptorceller), luktbulben och luktbarken. Denna avhandling handlar om utvecklandet av en modell av luktepitelet, lämpad för nätverkssimuleringar, i form av en grupp av luktreceptorceller (ORN) med olika sorters och mängder av luktreceptorer. Den utvecklade ORN-modellen är en encellsmodell med en låg abstraktionsnivå, med jonkanaler av Hodgkin-Huxley-typ. Genom att ta experimentella data som referens försöker jag i modellen representera en väl avvägd kompromiss mellan enkelhet och biologisk trovärdighet. A compartmental model of an olfactory receptor neuron Un modelo compartimental de una neurona receptora del olfato para su uso en modelos de grandes redes neuronales del sistema olfativo Resumen Para obtener modelos completos del sistema olfatorio es necesario desarrollar modelos de cada una de sus partes: el epitelio olfatorio (con neuronas receptoras del olfato), el bulbo olfatorio y el córtex olfatorio. Esta tesis trata sobre el desarrollo de un modelo del epitelio olfativo adecuado para simulaciones en red; en forma de una población de neuronas receptoras del olfato (ORNs) que expresan diferentes tipos y cantidades de receptores del olfato. El modelo desarrollado es el de una célula a muy bajo nivel de abstracción, incluyendo canales iónicos de tipo Hodgkin-Huxley. Tomando datos experimentales como referencia, este modelo intenta representar un buen equilibrio entre simplicidad y plausibilidad biológica. Un model compartimental d’una neurona receptora de l’olfacte pel seu ús en models de grans xarxes neuronals del sistema olfactiu Resum Per tal d'obtenir models sencers del sistema olfactiu és necessari desenvolupar models de cadascuna de les seves parts: l'epiteli olfactiu (amb neurones receptores de l'olfacte), el bulb olfactiu i el còrtex olfactiu. Aquesta tesi tracta sobre el desenvolupament d'un model de l'epiteli olfactiu adequat per simulacions en xarxa; en forma d'una població de neurones receptores de l'olfacte (ORNs) que expressen diferents tipus i quantitats de receptors de l'olfacte. El model desenvolupat és el d'una cèl·lula a molt baix nivell d'abstracció, incloent canals iònics del tipus Hodgkin-Huxley. Prenent dades experimentals com a referència, aquest model intenta representar un bon balanç entre simplicitat i plausibilitat biològica. A compartmental model of an olfactory receptor neuron Table of contents 1. Introduction .............................................................................................................................1 1.1. Motivation ...........................................................................................................................1 1.2. Project Goal .......................................................................................................................1 1.3. Thesis Structure ................................................................................................................2 2. Background .............................................................................................................................4 2.1. Overview of the olfactory system ...................................................................................6 2.1.1. The olfactory epithelium ...........................................................................................6 2.1.2. The olfactory bulb ......................................................................................................7 2.1.3. The olfactory cortex...................................................................................................8 2.1.4. Summary.....................................................................................................................8 2.2. Introduction to neurons ....................................................................................................9 2.2.1. Morphology .................................................................................................................9 2.2.2. Physical, chemical and biological processes ......................................................10 2.2.3. Olfactory Receptor Neurons (ORNs)....................................................................14 2.3. Introduction to modeling ................................................................................................15 2.3.1. The importance of modeling ..................................................................................15 2.3.2. The methodology .....................................................................................................15 2.3.3. Modeling the olfactory system ...............................................................................16 2.3.4. Problems about modeling ......................................................................................16 2.3.5. Parallel computing ...................................................................................................17 2.4. Introduction to NEURON, hoc and NMODL ...............................................................18 2.4.1. hoc .............................................................................................................................18 2.4.2. NMODL .....................................................................................................................18 3. The model...............................................................................................................................19 3.1 The base model................................................................................................................19 3.1.1 Why this base model?..............................................................................................20 3.1.2 Required changes ....................................................................................................20 3.2 Resulting model................................................................................................................21 3.2.1 Olfactory receptor machinery..................................................................................22 3.2.2 Calcium-activated potassium channel...................................................................24 A compartmental model of an olfactory receptor neuron 4. Methods of expressing the results..................................................................................25 4.1. FI-curve.............................................................................................................................25 4.2. Counting spikes for the construction of FI-curves .....................................................26 4.3. Expressing the odor input..............................................................................................27 5. Results ....................................................................................................................................28 5.1. Testing the initial model..................................................................................................28 5.2. Relation between gor and gKCa ......................................................................................33 5.3. Applying different odors to the same cell ....................................................................37 5.4. Bursting.............................................................................................................................39 5.5. Biologically implausible behavior..................................................................................41 5.6. Time varying input...........................................................................................................43 5.7. Visualization.....................................................................................................................48 6. Discussion and future work ..............................................................................................49 6.1. Discussion........................................................................................................................49 6.2. Future work ......................................................................................................................50 References .................................................................................................................................51 Bibliography...............................................................................................................................52 A compartmental model of an olfactory receptor neuron Acknowledgements First of all I would like to express my gratitude towards MSc Malin Sandström and Professor Anders Lansner, not only for their patient guidance during the project, but specially for giving me the opportunity to be involved in such an interesting project that has piqued my interest towards neuroscience and research. I want to thank them also for caring, not just professionally but also personally, I really appreciate it. Thanks to Ignasi, for the constant love and support given during these fleeting years, being involved during examinations and delivery periods, sharing with me the joy of triumphs and in the frustration of failures. To all fiber people who I spent time with, sharing lesson hours, project days, nights without sleeping, obstacles and succeeds. Very especially to Sabela, Jessica, Pablo and Francesc, for being much more than class-mates. To all friends I have met in Sweden, with who I have lived short but intensely these months. Very especially to the Flemingsberg-family, for making me feel so lucky of being at the end of the world with such special people. To the guy next-door for the long walks under different kinds of precipitation; to Dona, for sincere affection and care; and to Elvira for sharing with me her (bad) luck. And to Daniele, Sonja and new neighbors, for doing this last stressful period more enjoyable. I am also grateful to all this nice people of the department, some for assistance, some for distraction and some for both. Especially to David for help in developing, Antoine for help in Matlab and to Berhard for the interest and very helpful comments about the report. To my family for being there when I need them, especially to my brother, who has been my reference since always. And many thanks to my friend Alba, for being here even being thousands kilometers far away. A compartmental model of an olfactory receptor neuron Symbols and abbreviations Ca2+ Calcium ion EPSP Excitatory postsynaptic potential gKCa Parameter in the cell model defining the level of adaptation of the cell gor Parameter in the cell model defining the maximum conductance of the cell HH Hodgkin-Huxley Hoc Programming language (High Order Calculator) IPSP Inhibitory postsynaptic potential K+ Potassium ion Na+ Sodium ion NEURON Simulator environment for neural models NMODL Programming language OB Olfactory bulb OC Olfactory cortex OE Olfactory epithelium OR Olfactory receptor ORN Olfactory receptor neuron OS Olfactory System A compartmental model of an olfactory receptor neuron 1. Introduction This thesis reviews the work developed in the project “A compartmental model of a spiking and adapting olfactory receptor neuron for use in large-scale neuronal network models of the olfactory system”. This project is part of NeuroChem project [www.neurochem-project.org], an EU project that aims to develop computing paradigms and biometric artifacts to mimic the complete biological olfactory system, building computational models of its main blocks: the olfactory epithelium (OE) with olfactory receptor neurons (ORNs), the olfactory bulb (OB) and the olfactory cortex (OC). This work is being done at different levels of abstraction; in this thesis, the scope is limited to an olfactory epithelium model at a very low level of abstraction. 1.1. Motivation As mentioned above, the main aim is to create a computational model of a complete olfactory system. Going in this direction, some steps are already done. MSc Malin Sandström has set up several OB models of different sizes, she has also modeled the frequency response of a population of ORNs, using it as an input for her OB model. However, a model giving only a frequency response (a number) cannot describe features of the ORN response, such as response latency, response duration and adaptation. A better input that describes these lacking response aspects was required, the solution was modeling an ORN population. Even if there are several detailed ORN models that have already been published, they are too computationally consuming so they cannot be used for big arrays of ORNs in a large-scale olfactory system population. Therefore, we needed to create a simpler (i.e. less computationally consuming) ORN model for using it in a large-scale ORN array, as input to the OB model. Others in the same research group model the olfactory cortex at several different levels of abstraction. An abstract model of olfactory processing has also been constructed, and applied to real chemical sensor data. 1.2. Project Goal The goal of this Master's thesis project is to design and implement a large-scale model of a vertebrate ORN population. This should be done at a low level of abstraction, being aware of each single cell in the network and modeling them at a detailed level including ion channels. This single cell model has to be as simple as possible, but must still have a biologically plausible ORN behavior. The model should be a basic Hodgkin-Huxley model extended by adding a firing adaptation current and an olfactory receptor machinery. Each ORN should have an olfactory receptor (OR) type expressed, with different affinities to a certain number of odor compounds (odorants). In this way it will be possible to stimulate the neuron with different odors, even if they are a mixture of compounds. ~1~ A compartmental model of an olfactory receptor neuron Once the single cell is done, it has to be replicated creating a number of sets of olfactory receptor neurons expressing, each set, a different kind of OR. The model should be implemented in the NEURON simulator language, which allows running large network models in parallel. 1.3. Thesis Structure This thesis is structured as follows: Chapter 1 – Introduction. Sets the project in its context, introducing the objectives and giving reasons for the existence of this project. Chapter 2 – Background. Provides general information about different aspects related to the project. It includes: an overview of the olfactory system an introduction to neurons and specifically ORNs an introduction to computational modeling and, finally, a little information about the programming languages used to implement the model Chapter 3 – The model. I review here the development of the model. Speaking about the model taken as a base for this study, explaining the reasons of why we took this model as a base, discussing the required changes for obtaining the desired model behavior and, finally, describing the resulting model. Chapter 4 – Methods. In this chapter I introduce some useful vocabulary and describe the way the results are expressed in Chapter 5. Chapter 5 – Results. Presents the results of my experiments and an analysis of the evaluation of these results. They include: experiments for testing the initial model experiments for evaluating the behavior of the cells after tuning some of the cell parameters experiments varying the dose and the kind of the stimulus experiments showing bursting effects experiments getting non-desired behavior ~2~ A compartmental model of an olfactory receptor neuron experiments where we apply different ways to modulate the input and an illustration of how the experiments look in the visualization program Chapter 6 – Discussion and future work. I explain my conclusions and also propose possible direction for next steps. ~3~ A compartmental model of an olfactory receptor neuron 2. Background Olfaction is the sense that allows us to recognize smells in the environment. The odors are perceived by the receptor cells located in the olfactory epithelium, a mucous membrane lining in the upper nasal cavity. Even if one person can distinguish about ten thousand different smells (odorants1) [Lodish et al., 2008] it is often considered that the sense of smell in humans is poor. What is true is that the sensitivity, the discriminating power or the ability to distinguish between similar smells are better in some other animals than in humans. A reason that explains, at least in part, the low priority given to this sense, is that smell does not play an important role in human survival. Other animals depend heavily on the nose, allowing them to locate their partners or children, obtain food and escape predators. However, human olfaction is closely related to emotions and the subconscious, and plays an important role in social relations. Olfactory sensations are often confused with taste, since both are normally perceived at the same time. In fact, many foods are appreciated more by smell than by taste. Olfaction contributes to the initiation of the processes of digestion. When different odors reach the olfactory center of the brain, it sends the appropriate stimulus to the stomach to begin production of digestive juices, in this process also involved the vision, so that the presence of food stimulates production of saliva. Like taste, olfaction is a form of chemoreception. The olfactory system detects volatile and fluid-phase chemicals present in the air, for air-breathing animals, and in the surrounded aqueous medium, for water-dwelling organisms. These chemicals are odors and, even if normally they are found in very low concentrations, olfaction is very sensitive and only a few molecules are enough to stimulate the olfactory cells. Perceived differences in odor are attributed to different shapes and sizes of odor molecules that stimulate the olfactory sense. The smell is sensed by the olfactory system. As we will see in section 2.1, the olfactory system consists of three main components, the olfactory epithelium, the olfactory bulb and the olfactory cortex. Basically, the olfactory epithelium is the one which receives the odors from the environment and transfers this information to the olfactory bulb, which preprocesses and sends it to the olfactory cortex, which transforms this information in a sensation. Olfaction quickly adapts to the stimulus, which is believed to happen at different levels in the olfactory system. This phenomenon is called olfactory adaptation and it is very useful for filtering those stimulus which are constantly present as part of the environment. We will focus in the adaptation of the olfactory receptor neurons, at the epithelium level, when these cells become used to a particular odor, they cease to transmit it to the brain. 1 In this thesis we will consider an odorant as an odor compound. ~4~ A compartmental model of an olfactory receptor neuron Even if smell is the oldest of the senses, it follows the same principles for nearly all the animals, from small insects to big mammals. Despite not having the same origin, they had a common and parallel evolution that seems to mean that they are very well developed for the functions they have to perform. The specialized sensory cells that mediate olfaction are located in antennae and other parts of the body in invertebrates and in the nasal cavity in vertebrates. These specialized cells are (receptor) neurons and we will talk about them in future sections (section 2.2.3). The olfactory system is the least known of the five senses. The largest contribution that has been made on this issue was done by Linda B. Buck and Richard Axel, who were awarded the Nobel Prize in 2004. They discovered that in the mammalian genome there are approximately 1.000 different genes for olfactory receptors. Of these genes, only a portion is functional odor receptors, but each olfactory receptor is encoded by a different gene and can recognize different odorants. Humans have far fewer active odor receptor genes than other primates and other mammals. However, this does not seem to be the reason for the inferiority in olfactory ability. It is rather attributed to the proportion of olfactory epithelium compared to respiratory epithelium and its density (receptors per square centimeter). Axel and Buck also found out that each ORN expresses only a particular kind of olfactory receptor protein [Buck and Axel, 1991]. In turn, Peter Mombaerts discovered that input from all neurons expressing the same receptor is collected by only one dedicated glomerulus in the olfactory bulb. [Mombaerts, 1999]. Is it possible to copy all these mechanisms to equip a machine with this power? There are three main problems to solve: sensibility, scalability and stability. If we compare our advanced machines with the biological system we can see that in the three mentioned problems the biological system performs better. On the other hand, the biological system can poorly identify aroma compounds in complex mixtures (odors) while there are some sophisticated techniques that allow artificial systems to detect and identify them. Computational models are tools for understanding the neural system, since they allow us to integrate different levels of information. We can use them for finding out when we have missing or inconsistent information, for trying expensive or dangerous experiments and for generating experimentally testable hypotheses. The discovery of the genetic organization of the olfactory system together with experimental techniques give a firmer theoretical basis to the model. We will talk deeper about modeling in section 2.3. ~5~ A compartmental model of an olfactory receptor neuron 2.1. Overview of the olfactory system The olfactory system is the sensory system used to detect odors. This system is often considered, as well as the gustatory system, a chemical sense, both of them translate chemical signals to perception. The olfactory system has several purposes like to determine the representation and the concentration of an odor, distinguish between new smells in the background or identifying smells (relate them with the memory of what it represents). To carry out these functions, the system uses many brain areas. The olfactory system of vertebrate animals is divided in three main parts: the olfactory epithelium, the olfactory bulb and the olfactory cortex. 2.1.1. The olfactory epithelium The olfactory epithelium is where vertebrates sense smells. It is a specialized tissue located in the back of the nose, inside the nasal cavity, but in contact with air. Like other layers of epithelial tissue in the body, the olfactory epithelium contains a number of layers of cells. These cells include specialized neurons (olfactory receptor neurons, described in section 2.2.3), supporting cells (tall columnar cells that function as metabolic and physical support for the olfactory cells) and basal cells (stem cells capable of division and differentiation into either supporting or olfactory cells). The epithelium is covered by mucus with a complex composition, which among other functions, helps the ORNs to keep the right amount of ion concentrations. Molecules of odorants dissolve in this mucus and are detected by the olfactory receptors on the dendrites of ORNs. Different animals have varying degrees of sensitivity to smell, the larger the area covered by the olfactory epithelium, the more neurons, and the better the sense of smell. Animals that rely on their olfactory sense to alert them of the predators, potential food sources, or contamination in food or water have larger epitheliums. Dogs, for example, have 170 cm2 of olfactory epithelium while humans have 2-4 cm2 [Schild and Restrepo, 1998]. There is different kinds of ORNs depending on which receptor they express. Mice have approximately 1,000 different types of ORNs and approximately 10,000-20,000 cells of each type, this is, more or less, 10 millions cells in the epithelium [Wachowiak and Cohen, 2001]. The olfactory epithelium communicates with the olfactory bulb via the long axons of the olfactory receptor neurons within the olfactory nerve. In insects, smells are sensed by olfactory sensory neurons in the antenna, palps and tarsa and other parts of the insect body by the chemosensory sensilla. Odorants enter into the cuticle pores of chemosensory sensilla and get in contact with insect odorant binding proteins, before activating the sensory neurons. ~6~ A compartmental model of an olfactory receptor neuron 2.1.2. The olfactory bulb The olfactory bulb, or olfactory lobe, is a region of the central nervous system in vertebrates in which sensory input (coming from the OE) is interpreted, filtered and processed. We can differentiate two parts in the olfactory bulb: the main olfactory bulb that processes the ordinary odors, and the accessory olfactory bulb, which processes pheromones. The main olfactory bulb is laminated in six layers (listed from surface to the center): the nerve layer, the glomerular layer, the external plexiform layer, the mitral cell layer, the internal plexiform layer and the granule cell layer. Mitral cells and tufted cells carry the output from the bulb. Both are similar but the tufted cells are located more superficially, in the external plexiform layer. They have a main dendrite ending with a big tuft of glomerulus, and several secondary dendrites that end in the external plexiform layer for mitral cells, and in the olfactory cortex for tufted cells. The granule and periglomerular cells are inhibitory interneurons that regulate the activity of the principal neurons. The point where axons of the ORNs synapse with the dendrites from mitral, tufted and periglomerular cells is called glomerulus. Each glomerulus receive input from several ORNs but all of them express the same olfactory receptor. Glomeruli are small, about 20-200 micrometers of diameter, and more or less spherical depending on the species. They form the glumerular layer, which is one or two glomeruli thick. The information about smell is contained in the combination, the relative intensity and, probably, also in the spatial distribution of the activated glomeruli. The latencies of different glomeruli to the same stimulus may also contain odorant information. The activation pattern of the glomeruli changes when the concentration of an odorant varies, so the perception of an odor can completely change with the amount of concentration. When the bulb receives the input from the epithelium it does it through the mentioned connections existing in the glomeruli. The mitral and tufted cells send the output to the pyramidal cells in the olfactory cortex. However, the OB also receives top-down information from higher brain areas. The potential functions that the OB is supposed to perform are: improvement in discrimination between odors improvement in sensitivity and noise suppression filtering and suppression of background, transmitting only important information However, it is not clear which of them are performed exclusively by the OB because, as mentioned, it needs feedback from higher areas of the brain. ~7~ A compartmental model of an olfactory receptor neuron The analogous organ of the olfactory bulb in insects is the antennal lobe, where the first processing of the smells is done. 2.1.3. The olfactory cortex The olfactory cortex is the region of vertebrates brain where the smell information coming from the OB is analyzed and converted into a perception. It consists of some different areas, the most important of them is the piriform cortex (or primary olfactory cortex). The main neurons in the piriform cortex are the pyramidal cells and it is laminated in three different layers: 1st layer. Contains the axons and association fibers that connect pyramidal cells 2nd layer. Contains the cell bodies of superficial pyramidal cells 3rd layer. Contains the cell bodies of deep pyramidal cells, interneurons and connection fibers. Another area in the OC is the anterior olfactory cortex, which is located between the olfactory bulb and the piriform cortex and is strongly connected to them. It seems to contribute to the transmission of information between them in both directions, but its function is still unclear. Hierarchically, the homologous of the olfactory cortex in insects are the mushroom body structures. 2.1.4. Summary In vertebrates, the main olfactory system detects odorants that are inhaled through the nose. There, they contact the main olfactory epithelium, which contain olfactory receptor neurons. Olfactory receptor neurons transmit the signal through the olfactory nerve to the glomeruli in the olfactory bulb. By the combination of the activated glomeruli the olfactory bulb defines an output which is sent by the mitral/tufted cells to the pyramidal cells in the olfactory cortex, which process them for the formation of perception. ~8~ A compartmental model of an olfactory receptor neuron 2.2. Introduction to neurons Neurons are a type of nervous system cells whose main characteristic is its plasma membrane excitability. They are specialized for the reception of stimuli and conduction of nerve impulses (called action potentials or spikes) between themselves or to other cell types, as for example the muscle fibers of the motor plate. Highly differentiated, most neurons do not divide after reaching maturity, however, a minority of neuron types, like the olfactory receptor cells, does. 2.2.1. Morphology A typical neuron consists of: a voluminous central nucleus, located in the soma, a perikaryon2 that contains the organelles typical of any eukaryotic cell and neurites (i.e., usually an axon and several dendrites) that emerge from the perikaryon. The characteristics and functions of each part are described below. Cell body, soma or pericaryon It is the bulbous part of the neuron. It contains many different organelles in the cytoplasm surrounding the nucleus. The most important organelle is the Nissl substance where the most of the protein synthesis is done. The nucleus usually occupies a central position in the cell body and it is very conspicuous, especially in small neurons. It contains one or two prominent nucleoli and dispersed chromatin, which suggests the relatively high transcriptional activity of this cell type. The nuclear wrapper, with many nuclear pores, has a highly developed nuclear lamina. Dendrites One or more short extensions usually receive synaptic contacts from other neurons and transmit impulses toward the cell body. They are branches coming from the neuronal soma cytoplasmic projections enveloped by a plasma membrane without myelin wrapped. Sometimes they have an irregular contour, developing spines. Axon A long extension of neuronal soma coated by one or more Schwann cells in the peripheral nervous system of vertebrates. It conducts impulses from the cell body to another neuron or target organ. 2 From greek, peri = around, near + karuon = nut. ~9~ A compartmental model of an olfactory receptor neuron Figure 2.1: Schema of a standard neuron, showing its main parts. The image is in the public domain (Wikimedia Commons) 2.2.2. Physical, chemical and biological processes 2.2.2.1. Nerve impulse Neurons transmit electric waves produced following a transient change in permeability in the membrane. The spread of these waves is due to the existence of a potential difference (that exists because of the different ion concentrations on both sides of the membrane) between the inner and outer surface of the cell. The charge of an inactive cell remains in negative values and varies within narrow margins. When the membrane potential of an excitable cell is depolarized beyond a certain threshold the cell generates an action potential. The cell membrane contains a protein pump called the sodium-potassium pump. “Three sodium ions from inside the cell first bind to the transport protein. Then a phosphate group is transferred from ATP to the transport protein causing it to change shape and release the sodium ions outside the cell. Two potassium ions from outside the cell then bind to the transport protein and as the phosphate is removed, the protein assumes its original shape and releases the potassium ions inside the cell.” [Doc Kaiser's Microbiology Website: http://student.ccbcmd.edu/~gkaiser/goshp.html] ~ 10 ~ A compartmental model of an olfactory receptor neuron In the membrane there are also sodium and potassium ion channels that allow sodium ions to leak in and potassium ions to leak out. The resting membrane potential is the balance of sodium and potassium ions reached because of these pump and ion channels. When an action potential occurs the membrane potential changes for a while. An action potential consists of two phases: depolarization and repolarization. A stimulus can make the membrane potential change a little. If the membrane potential changes enough to pass the threshold, the depolarization starts, the voltage-gated sodium channels open and sodium enters into the axon, causing a positive zone in the axon. The repolarization starts when this positive region makes the surrounding sodium channels close. Then potassium channels open, and let potassium ions out. This makes the inside of the cell negative again, so the charge across the membrane returns to its initial state. This process of depolarization and repolarization continues along the axon as a chain reaction. Then the sodium-potassium pump restores the ion concentrations. 2.2.2.2. Hodgkin - Huxley model The standard Hodgkin - Huxley model of an excitatory neuron represents the biophysical characteristic of its membrane. The current in a segment of nerve membrane, Im, is obtained from Ohm's law: where Vm denotes the membrane voltage, Cm is the membrane capacitance, IK is the potassium current, INa is the sodium current and IL is leakage current carried by other ions that move passively through the membrane. These currents are defined by the equations: ; ; ; where Gk is the conductance (1/Rk) and Ek is the equilibrium potential for each ion. A gate in the membrane has an intrinsic resistance and the cell membrane itself has an intrinsic capacitance. There are sodium gates, potassium gates and, in addition, there are other ions that move across the membrane because of pumps, other gates, etc. (not described by the original HH model). This additional ion current is primarily carried by chloride ions and it is modeled as a leakage current with its own resistance. Each ion has its own equilibrium potential which is determined by applying the Nernst equation. In the equivalent circuit (Figure 2.2) current going across the membrane has two components, the charge of the membrane capacitance and the movement of ions across the membrane (INa, IK and IL). ~ 11 ~ A compartmental model of an olfactory receptor neuron Figure 2.2: Basic components of Hodgkin–Huxley-type models. The membrane is represented as a capacitance (Cm). Voltage-gated and leak ion channels are represented by conductances (Gk). The electrochemical gradients driving the flow of ions are represented by batteries (Ek). The different conductances GNa, GK and GL are the result of the combination effect of many microscopic ion channels, each one containing a small number of gates. Each gate can be in two states, open or closed. When all the gates in a channel are open, then the channel is open and otherwise the channel is closed. Defining pi as the probability of a gate of type i to be open, pi depends on the membrane voltage: where αi and βi are voltage-dependent rate constants describing the transition rates between closed-to-open and open-to-closed state, respectively. When an individual channel is open, it contributes to the conductance. The equation that defines the conductance of each type of channel k is: where gk is a constant that determines the maximum possible conductance when all channels are open. In the sodium channels we have three m gates and one h gate and in the potassium channel we have four n gates, applying the previous formula to our specific channels we get: ; ~ 12 ~ A compartmental model of an olfactory receptor neuron where pm=m, is the probability of a gate of type m to be open and similarly for h and n. Summarizing we have: 2.2.2.3. Signal transmission between neurons There exist two kinds of connections between neurons, they are electrical gap junctions and chemical synapses. Electrical gap junctions directly connect the cytoplasm of two cells, which allows various molecules and ions to pass between cells. Chemical synapses are functional connections between neurons or between neurons and non-neuronal cells. Broadly speaking, there is one neuron (the presynaptic cell) that releases a neurotransmitter into a small space (the synaptic cleft) adjacent to another cell (the postsynaptic cell). In more detail, a synaptic transmission event starts when an action potential traveling along the presynaptic cell reaches the synapse. The depolarization of the membrane at the synapse makes calcium channels open so calcium ions flow inside the cell increasing the calcium concentration. It activates the calcium-sensitive proteins that gate the membrane-bound spheres (synapse vesicles) containing the neurotransmitters, making them open and dropping their content into the synaptic cleft. Some of the neurotransmitters bind to the chemical receptor molecules located in the membrane of the postsynaptic cell, what produces on the later an excitatory postsynaptic potential (EPSP) or an inhibitory postsynaptic potential (IPSP) depending on what channel type is opened. An EPSP is the depolarisation of postsynaptic membrane potential as a result of opening the ligand-sensitive channels. An EPSP is caused by a flow of ions called excitatory postsynaptic current and, normally, it is the flow of positive ions into the cell, but it could also result from a decrease in outgoing positive charges. An IPSP is the opposite process, thus it causes hyperpolarisation and is the result from the flow of negative ions into the cell or positive ions out of the cell. Each synaptic input often gives only a small depolarization so many inputs must cooperate to reach the threshold to fire an action potential. However, both EPSPs and ISPSs are graded so larger inputs result in greater probability for that the cell fires an action potential. Amino acid glutamate and GABA are the most common neurotransmitters associate with EPSPs and IPSPs, respectively. ~ 13 ~ A compartmental model of an olfactory receptor neuron 2.2.2.4. Neural networks A neural network is defined as a population of physically interconnected neurons or a group of single neurons that receive signals processed in the manner of a recognizable circuit. The communication between neurons means that, once a neuron is excited above a certain threshold, it transmits a signal through its axon that excites or inhibits the surrounding neurons, and so on. 2.2.2.5. Firing adaptation Most of the sensory receptors adapt to a constant stimulus. Neural adaptation can be described as the decrease over time in the responsiveness of the receptor neuron, that is, the frequency of spikes decrease or even stops. Adaptation may result out of characteristic response properties of the receptor neuron’s membrane, such as inactivation of Na+ and Ca2+ channels or activation of a calcium-dependent K+ channel. It may also depend on the nonneural accessory cells that surround the receptive ending of the primary sensory neuron. The calcium current is activated when an action potential occurs, letting some calcium ions go inside the cell, which makes the amount of calcium inside the cell increase. This is a slow process, but when the amount of calcium reaches a determinate level, it makes the calcium-activated potassium channel open. That lets more potassium going out of the cell causing hyperpolarization and makes the cell spikes slower. The calciumdecay current makes the amount of calcium inside the cell decrease. 2.2.3. Olfactory Receptor Neurons (ORNs) In vertebrates, ORNs reside on the olfactory epithelium, in the nasal cavity. Axons from these cells converge into glomeruli, in the olfactory bulb. Similarly, in insects, ORNs are located in the antenna and their axons converge in the antennal lobe. Even if ORNs in both vertebrates and insects are very similar, we will focus on vertebrate ORNs. ORNs are bipolar neurons with a dendrite ending in a knob with ciliary protuberances that lie inside the mucus in the nasal cavity. Each ORN expresses only one kind of olfactory receptor (OR), randomly chosen, and the ORs are located in the ciliary membrane. Axons of ORNs expressing the same receptor end in the same glomerulus of the bulb. The ORN can respond to a certain number of odorants and, like most of the sensory receptor neurons, they adapt with constant stimulus. The regeneration of ORNs is one of the few cases of adult neurogenesis in the central nervous system. The epithelium is continuously exposed to toxic substances and mechanical damage. For this reason ORN are replaced approximately every 70 days by basal cells [Sandström, 2010]. The biochemical process taking place in the receptor neurons is described in section 2.2.2. ~ 14 ~ A compartmental model of an olfactory receptor neuron 2.3. Introduction to modeling Among many other benefits, modeling is useful to improve understanding of complex systems. The main goal is that the model behaves in the same way as it would do if it was a real system. One of the central questions is keeping the model as simple as possible, creating a conceptual model that expresses what we know about the system while omitting unnecessary details. 2.3.1. The importance of modeling Understanding the nervous system requires knowledge about many things like anatomy of the neurons and pathways, pharmacology of ion channels, biochemistry of enzymes and genes, etc. All this information is necessary for understanding, but not enough. The chemical signals that carry out the processing of information in the brain are distributed in space and time. The processes that regulate these signals are so complex that they cannot be evaluated just by intuition, thus understanding requires empirically based modeling. 2.3.2. The methodology There are two important steps involved in the way to pass from a physical system to a computational model. The first step is to formulate a conceptual model and the second one is to implement it. Creating a conceptual model consist in capturing the essential features that underlie a particular function or property of the physical system, omitting real-world complexities. If the real system is complex it may require simplifying and abstracting but if the system is simple enough, there is no reason for further simplification. Usually conceptual models are expressed in mathematical form but sometimes they are also expressed in the form of a computer algorithm. To evaluate a non-intuitive model, it is necessary to compare its behavior with a prediction. This prediction can be, for instance, a hypothesis or a test performed by a computational model, but it can also be experimental data. The computational model is the application of the conceptual model as a computer simulation. The main aim of this step is that the computational model has to be as faithful to the conceptual model as possible, neither introducing new properties, nor additional simplifications. Computational models are useful for testing hypothesis, working as a virtual laboratory [Carnevale and Hines, 2006]. ~ 15 ~ A compartmental model of an olfactory receptor neuron Figure 2.3: How we create a simple conceptual model from a complicated physical system by simplification. Most of the anatomical complexity of the physical system lies in the dendritic tree, but the conceptual model approximates it by a cylindrical cable. The computational model, written in hoc, implements exactly the conceptual model. 2.3.3. Modeling the olfactory system The olfactory system can be modeled at different levels of abstraction. It can be done from a very low level, being aware of each cell, and all of its compartments with its parameters; until higher levels of abstraction like algorithms that imitate complete network systems, seeing them as a black box. One should be aware about the necessities (for what is this model going to be useful?) and also about the available computational resources. The Hodgkin-Huxley model requires a quite low level of model abstraction, which implies that is more computationally consuming when the size of the network increases. There are other more abstract neuron models such as Lapicque, McCulloch-Pitts or FitzHugh-Nagumo [Sandström, 2010]. 2.3.4. Problems about modeling One of the big problems when trying to model the olfactory system is the lack of information about the different kinds of cells, projections and connections. Another big problem when modeling the olfactory system is the large number of cells. Each cell is a complex system that can be connected to thousands of other cells. Therefore, we are talking about large-scale networks with multitude of synapses produced between cells. This amount of data makes it impossible to process it in an average personal computer. The obvious way to solve these problems is making the models as conceptual as possible, using the minimal number of different types of cells and with small connectivity detail. We can do so by generalizing the cell and/or connection model. Once it is not possible to synthesize the model any further (or it may result in a loss in the level of realism desired), it is possible to parallelize the processing to run in a cluster of computers. ~ 16 ~ A compartmental model of an olfactory receptor neuron 2.3.5. Parallel computing The main principle of parallelization is that large problems can be divided into smaller ones, and then, each of these smaller problems can be solved independently. In computing it can be easily applied, as each problem is a (normally huge) set of simple calculations, so that many processors can carry them out simultaneously. Of course, not all the calculations can be done in parallel, as there might be dependencies between them and, typically, communication and synchronization between different subtasks are big obstacles to get a good performance. Parallel computer programs are also more difficult to write than sequential ones. However, for network models there exists programming languages, like NEURON language (see section 2.5), that allow to implement easily large network models for being run in parallel. Such parallelism is essential for the existence of large-scale network models. ~ 17 ~ A compartmental model of an olfactory receptor neuron 2.4. Introduction to NEURON, hoc and NMODL NEURON is a simulation environment for neural models, which allows us to simulate from a model of an individual neuron to a model of a large network of neurons. The greatest advantages compared to other generic simulators are that it allows including extracellular potential close to the membrane and involves many ion-specific channels and ion accumulation. NEURON is designed around the notion of continuous cable sections, which are connected together to form any kind of branched cable, and with properties that vary with position along the section. Membrane properties can be defined by expressing models in terms of kinetic schemes and sets of equations. Typically, one should write NEURON programs with the specialized hoc language, but since 2006 Python was added as an alternative interpreter. With both languages it is possible to take advantage of multiprocessors or workstation clusters in order to accelerate processing. Apart from that, it is possible to add mechanisms such as voltage and ligand-gated ion channels, diffusion, etc. using NMODL [Carnevale and Hines, 2006]. 2.4.1. hoc hoc is an acronym for High Order Calculator. hoc is an interpreted programming language with a C-like syntax that has been extended to include functions specific to the domain of modeling neurons, implementing a graphical interface and object oriented programming. hoc is based on the floating point calculator developed in [Kernighan and Pike, 1984]. Its basic functionality was to evaluate floating-point numerical expressions, but then, variables were added, conditionals, loops, user-defined functions, simple IO, and more. NMODL allows us to extend the functionality of hoc. 2.4.2. NMODL NMODL is a high level language with a syntax very close to mathematical and chemical notation. This very compact specification makes it easier for the user to keep focused on the model, and not on programming. The language was created by Michael Hines as a descendant of MODL and has its same basic syntax and style of organizing the source code into named blocks. NMODL provides NEURON the means to incorporate indispensables biophysical mechanisms for modeling the neuronal function in the model. The first step is writing the code in a text file with .mod extension. It should describe a mechanism as a set of nonlinear algebraic equations, differential equations, or kinetic reaction schemes. This text is translated to C code by the translator and then compiled by the compiler. ~ 18 ~ A compartmental model of an olfactory receptor neuron 3. The model This is the review of the development of the model of the olfactory epithelium for network simulations, a set of olfactory receptor neurons expressing different kind and amount of olfactory receptors. First I will explain the model as a single cell, because this is the elementary building block for the epithelium model. Taking Rospars [Rospars et al., 2003] results as a reference we tried to develop a neuron model that represents a good compromise between simplicity and biologically plausibility. The number of olfactory receptor types in small vertebrates such as rats is up to 1000 and the number of neurons of each type is about 5000. In our model we adjust these numbers, to make it possible to run it on an average personal computer, but these numbers can easily be increased if we have a powerful enough computer. As there are many published models available in model databases on the Internet, we decided not to start from zero but get one of these models as a base for ours. After studying the basic working principle, we changed and adapted it to make it fit better to the physiological data from vertebrate ORNs. We started with a simple model and made it more detailed matched to data on vertebrate olfactory receptor neurons. 3.1 The base model We took the model available in the ModelDB webpage [http://senselab.med.yale.edu/ ModelDb] called “Hodgkin-Huxley models of different classes of cortical neurons”. This model is described in [Pospischil et al., 2008], where they reviewed the development of four models of the most prominent electrophysiological classes of neurons inspired from the classification of Connors and Gutnick (1990). These classes are “fast spiking”, “regular-spiking”, “intrinsically bursting” and “low-threshold spike”. For each class they took the minimal set of voltage-dependent currents to account for the data. To obtain very generic models they used data from different preparations in vivo and in vitro. Between these four models we decided to take the “regular-spiking” neuron model, the reasons of this choice are explained in section 3.1.1. They focus on obtaining Hodgkin-Huxley type models, capturing the essential features of the intrinsic properties using a minimal number of voltage-dependent conductances. The models should represent the main intrinsic firing and response properties of excitatory and inhibitory neurons. The membrane equation in all the models is described by the following formula: where V is the membrane potential, Cm = 1 m F/cm2 is the specific capacitance of the membrane, gleak is the resting (leak) membrane conductance, Eleak is its reversal potential. INa and IKd are the sodium and potassium currents responsible for action potentials, IM is a slow voltage-dependent potassium current responsible for spike~ 19 ~ A compartmental model of an olfactory receptor neuron frequency adaptation, IL is a high-threshold calcium current and IT is a low-threshold calcium current. The four above mentioned neuron classes have different realizations of the specific currents. More details about each voltage-dependent current can be found in point 2 (Methods) of [Pospischil et al., 2008]. 3.1.1 Why this base model? We used different decision criteria when we had to choose the base model. The neuron model we were looking for should be a generic single compartment spiking Hodgkin-Huxley type model. It had to have an as basic setup as possible, with just the needed basic currents. However, we were looking for a model that had an already tested spike-frequency adaptation current in addition to the basic HH currents, since we want to introduce spike frequency adaptation later on. We took in consideration the existence of a publication which described the model in detail. And finally, it was essential that the model was implemented using NEURON, as it was the simulation environment that we wanted to use for our implementation. We chose NEURON as simulation platform because it is very suitable to simulate detailed neuron models as well as large-scale networks in parallel, which is essential for a later scaling-up of the number of neurons. The channels implemented in this basic model are very similar to the ones we needed in ours. It has the basic HH currents and also an M current giving the cell the ability of frequency adaptation. We checked the behavior of this model before and after adding the olfactory receptor machinery (for description of the OR machinery see section 3.2.1) and the results were similar to those of Rospars. But after some experiments, we realized that the M current did not fit exactly with our expectations (see below, section 3.1.2 Required changes). Finally, it was also important for the later scaling up of the network size that the cell shape was very simple, just a cylindrical soma, building a single-compartment cell. 3.1.2 Required changes After some experiments we realized that the adaptation current did not behave how we expected. Trying to tune the adaptation current for having a certain point of saturation (see section 5.2 describing our goal), we realized that it made the firing threshold move as well. This unwanted behavior did not fit with the input the olfactory bulb model expects, so we decided to change the M current to a calcium-activated potassium channel, which is proved to exist in ORNs. We took an existing channel model from [Moczydlowski and Latorre, 1993] available in ModelDB, which is explained in detail in section 3.2.2. Adding this calcium-activated potassium channel required also the addition of a calcium current and this, in turn, required a calcium-decay current. The calcium current was already inserted in the base model (IL), but it was necessary to change some of the ~ 20 ~ A compartmental model of an olfactory receptor neuron default parameters to obtain the suitable behavior. The calcium-decay current we added was written by [Destexhe et al., 1995] and it is available in the ModelDB website. We also had to add, of course, the olfactory receptor machinery. This current is described in detail below (section 3.2.1). Another difference with the base model was the kind of input. While in the base model the input was an injected current directly by an electrode, our model should receive the representation of an odor as input. The different amounts of odorants that make up the odor are read from a file by the model. After all these processes the original code was substantially changed but the HH channels (sodium and potassium) remain intact. 3.2 Resulting model At the end, the cell model that we implemented has one single compartment, the soma, with some currents and channels: Na+ and K+ currents, responsible for the action potentials KCa current, responsible for the adaptation Ca2+ current, responsible for the bursting and the increase of calcium concentration OR current, responsible for receiving the stimulus and cause the initial current Ca2+ decay current, responsible for keeping the calcium concentration in a suitable level The behavior of the model is like a basic HH model including only sodium and potassium currents but extended with additional currents: the firing adaptation current and the olfactory receptor machinery. Figure 3.1: Schema of the cell model. It also has calcium decay (not shown in picture). The dendrites here are only for decoration. ~ 21 ~ A compartmental model of an olfactory receptor neuron The cell receives an input consisting of ten values, referring to the amounts of concentration of the ten odor compounds that we consider in the model. These values are treated by the olfactory receptor machinery (see below, section 3.2.1) having a current as a result. This current makes the membrane potential change, so, if the stimulus is high enough, the depolarisation starts and following the typical action potential process (see section 2.2.2.1), a spike occurs. After some spikes the adaptation starts to take place (see the biochemical description of the adaptation process in section 2.2.2.5 and description of the channel in section 3.2.2) and the spiking frequency decreases. At the end, the membrane equation is described by the following formula: where most of the parameters are the same as in the equation of the original model (see section 3.1), with the addition of IKCa, the potassium current gated by the calcium activated potassium channel and responsible for spike-frequency adaptation, and IOR, the current generated by odorants binding to olfactory receptors (described in section 3.2.1). The computational model has been developed and run in the NEURON simulation environment [Carnevale and Hines, 2006]. The analyses of the results have been done using Matlab and the program for the visualization of these results has been done in OpenGL. 3.2.1 Olfactory receptor machinery The olfactory receptor machinery is responsible for translating the input into the first amount of current. In this model the odor stimulus arriving to the cell is a mix of ten ligands. Then the input of the cell will be ten values, Ml, meaning the amount of each ligand l. The cell model has one array of ten values that store the KD values. The KDl value can be described as the affinity of the olfactory receptor to a determined ligand l. Then each amount of ligand Ml is combined with each KDl by the formula: where h is the Hill coefficient, which determines the degree of cooperativeness of the ligand binding to the receptor. In our model we have noncooperative reactions (ergo h=1). This means that the affinity of the receptor for a ligand does not depend on if other ligand molecules are already bound. Then the current is described by: and ~ 22 ~ A compartmental model of an olfactory receptor neuron where gor is a parameter of the cell that defines the maximum conductance when all receptor gated channels are opened and gri is the conductance. V is the membrane voltage and EOR is the equilibrium potential (0 mV). The olfactory epithelium model (set of single cell models) has different receptor types, all the cells of the same receptor type share the same array of KD values while gor value can be different for each cell. Applying the current immediately would mean an instantaneous increase in the current amplitude. Firstly, this is unrealistic, since the stimulus increase is a continuous process in natural ORN, and secondly, this would cause unrealistic response in the model. So we defined some functions for tuning the input simulating the natural breathing. The two functions applied were: 1) slow incoming of the stimulus with a sigmoidal function Figure 3.2: Input of the stimulus defining a sigmoid function ~ 23 ~ A compartmental model of an olfactory receptor neuron 2) simulating the natural breathing of a rat (200 ms per period) sinusoidal function Figure 3.3: Input of the stimulus defining a sinusoidal function 3.2.2 Calcium-activated potassium channel The calcium-activated potassium channel is the responsible for the adaptation. The main formula that describes the current is: where gKCa is the maximum conductance of the channel, oKCa is the fraction of open channels, V is the membrane voltage and EKCa is the equilibrium potential. More details about this channel can be found in [Moczydlowski and Latorre, 1993]. ~ 24 ~ A compartmental model of an olfactory receptor neuron 4. Methods of expressing the results 4.1. FI-curve Some of the results of the experiments are expressed using frequency-intensity curves (FI-curves). FI-curves are the way of representing frequency of spikes depending on the intensity of the input that the cell receives. Normally we observe three important parts in FI-curves: the threshold of spiking, the dynamic range and the saturation point. The threshold of spiking is the amount of intensity for which the cell starts spiking. The dynamic range of the input is the firing frequency range over which a change in the input leads to a change in the neuron's output frequency. The dynamic range is limited from below by zero and from above by saturation. And the saturation point is the first point of the curve that reaches the maximum frequency [Shin, 2001]. Figure 4.1: Example of FI-curve showing the main parts: spiking threshold, dynamic range and saturation point ~ 25 ~ A compartmental model of an olfactory receptor neuron 4.2. Counting spikes for the construction of FI-curves When the spiking rate of a neuron is not the same over time, it is a matter of judgment, which spikes to count for a FI-curve. We regard the spike frequency at stimulus onset and when the cell is in a steady state, i.e. the firing frequency does not rapidly change anymore. Figure 4.2: Example of experiment result showing what has been considered as the initial and the sustained state For measuring the frequency at the beginning of the stimulus (initial state) we count the spikes occurring in the first half second, when normally the activity is higher. For measuring the frequency when the sustained state is reached, we took the spikes in the third second into account, when the cell is always spiking regularly. Figure 4.2 shows what is considered the initial and the sustained state. The formula applied is: As in the example, most of the experiments have a duration of three seconds, however the time is always printed in spiking response figures. Note: the threshold for the spike detection was set to 20 mV, i.e. when the membrane potential crosses this threshold we counted this as a spike. ~ 26 ~ A compartmental model of an olfactory receptor neuron 4.3. Expressing the odor input In most of the experiments, the input is some amount of odor. If this amount is shown in the axis of a graph in the results, normally the axis is called “Amount of odorant (factor of KD expressed in log)”. Being x the number in the axis, the formula applied is: where factor expresses how many times the amount is bigger (or smaller, if x is negative) compared to the KD value. For example, if the KD value expressing the affinity of the observed cell to the applied odorant is 10-7 and the value on the axis is 2, then the total amount of odorant applied is 10-7 * 102 = 10-5 and it means that the amount of input is one hundred times bigger than the KD value. ~ 27 ~ A compartmental model of an olfactory receptor neuron 5. Results 5.1. Testing the initial model 5.1.1. Injected current The first step was checking how the model worked before inserting the olfactory receptor machinery. We wanted to check the firing rate of the single-cell model. For doing that we put an electrode to the cell and inject current into it for three seconds. We ran the simulation several times, varying the intensity of the injected current each time and observing the number of spikes at the initial and sustained state. As we can see in Figure 5.1, both frequencies grow while the current increases, but frequency increases quicker at the initial state than it does at the sustained state. This is because of the adaptation, which makes cells spike slower in the sustained rate. Figure 5.1: Firing rate of one neuron depending on the intensity (current from 0.4 to 3.175 nA) ~ 28 ~ A compartmental model of an olfactory receptor neuron Figure 5.2 shows how the latency of the first spike is modified in relation with the amount of current that is injected in the cell. Logically, as we put more current on it, the latency of the first spike decreases. Figure 5.2: Latency of the first spike of one neuron depending on the amplitude (current from 0.475 to 3.175 nA) These results agree with the ones written in [Rospars et al., 2003] as we can see in Fig. 4 b, of that paper. The curves shown in the figure express the latency of the first response of one neuron at different doses of odorant and they have a similar shape to Figure 5.2 of our paper. This is consistent with our results because, a higher intensity of current corresponds to higher dose of odorant. But, as it is discussed in section 6.1, the latencies are much longer in Rospars’ results. ~ 29 ~ A compartmental model of an olfactory receptor neuron 5.1.2. Odor stimulus We repeated the same experiment as in section 5.1.1 once the olfactory receptor machinery was already implemented. In this case the input was, of course, the representation of an odor. Therefore, in each simulation we increased the amount of odorant stimulus applied as an input. The difference to the previous experiments is that now the input was not simply a current, but the current was calculated with the formula in section 3.2.1 describing the olfactory receptor machinery. We plotted the frequency curves for initial and sustained states, and again, we could see how the initial state curve grows quicker than the sustained state curve because of the adaptation (Figure 5.3). Figure 5.3: Firing rate depending on the amount of stimulus ~ 30 ~ A compartmental model of an olfactory receptor neuron In Figure 5.4 we can see how the latency draws a similar curve as in the experiment before. The higher the concentration of input is, the sooner it fires. Figure 5.4: Latency of the first spike depending on the amount of stimulus Figure 5.5 and Figure 5.6 show how voltage evolves over time applying different amounts of odorant to the same cell. In the first figure the stimulus is lower and therefore the number of spikes is smaller. In these figures one can clearly notice the effect of the adaptation, spikes at the beginning of the input are fast but they gradually decelerate until they reach the sustained state. ~ 31 ~ A compartmental model of an olfactory receptor neuron Figure 5.5: Spiking response of a cell with gor=0.0002 and gKCa=0.005, when an odorant is applied in concentration=1.13 (KD*101, 13) Figure 5.6: Spiking response of a cell with gor=0.0002 and gKCa=0.005, when an odorant is applied in concentration=1.47 (KD*101,47) ~ 32 ~ A compartmental model of an olfactory receptor neuron 5.2. Relation between gor and gKCa We wanted to see how much we could increase the range value for gor. As seen in section 3.2.1, gor is a parameter of the cell that determines the maximum conductance when all receptor gated channels are opened. We repeated the experiment with several cells, each one with different gor values. We realized that for small gor, the curves reach the saturation state, where the frequency remains stable even for an increase in the amount of stimulus. This is because, at the saturation point, all the channels are already open and the maximum level of conductance is reached. For larger values of gor, the saturation is not reached within the investigated range, arriving to get very high values of frequency. When these high values of frequency are reached, usually the cell starts to behave in an unrealistic way. We did not regard these cases since we considered they reflect a biologically implausible behavior (this kind of spikes is described in section 5.5). Figure 5.7: Firing rate depending on the amount of stimulus. The different curves correspond to cells with different gor, the values go from 0.0001 to 0.8192, doubling it for each curve following the direction of the arrow. The frequencies are the values in the sustained state of the cells. ~ 33 ~ A compartmental model of an olfactory receptor neuron We wanted to reach a common saturated point value for all the cells in the experiment. This could be achieved by tuning the value of the gKCa parameter in calcium-activated potassium channel (responsible of the adaptation, see section 3.2.2). Increasing this value, the adaptation of the cells gets stronger, what makes the frequency in the saturated state decrease. Then with the same amount of odorant, all the channels will be opened and the maximum conductance will be reached but the frequency (in the sustained state) will be smaller. Figure 5.8 shows this influence of gKCa on the cells. Figure 5.8: Firing rate depending on the amount of stimulus. The different curves correspond to cells with same gor but different gKCa, showing the influence of gKCa on the cell. With gKCa=0.1 saturation point is reached at 2 Hz, while with gKCa=0.0005 it is reached at 148 Hz. The frequencies are the values in the sustained state ~ 34 ~ A compartmental model of an olfactory receptor neuron We fixed a suitable (i.e., biologically reasonable) saturated frequency (20 Hz), where all the curves must stop increasing. For small gor values it was possible to find suitable gKCa values so that the cell showed the desired saturating behavior. But again, the cells with higher gor never reached the saturation point because before that, the nonrealistic behavior occurred. The point where this behavior starts is shown in the curves as a small circle, meaning that after this value we did not regard the results for considering them unrealistic. Figure 5.9: Firing rate depending on the amount of stimulus. The different curves correspond to cells with different gor and gKCa values. The frequencies are the values in the sustained state of the cells ~ 35 ~ A compartmental model of an olfactory receptor neuron After finding the gKCa values for which the cell shows saturating behavior for some of the curves, we could establish a relation between the value of the gor and the value of the gKCa as is shown in the Figure 5.10. Figure 5.10: Relation between the value of the gor and the value of the gKCa The figure suggests that the relation between the increment of gor and the corresponding need to increase gKCa is not linear but as gor grows, gKCa needs to grow less. Since only few values were used, this figure shows only the qualitative relation, which is sufficient for the simplified model. ~ 36 ~ A compartmental model of an olfactory receptor neuron 5.3. Applying different odors to the same cell The next two figures show the FI-curves of fourteen cells, all of them with different gor values but same affinity to odorants. The cells with same numbers in both experiments are exactly the same, but in the experiment of the first picture we applied a different odorant than in the second one. As we can see the amount of odorant required for making them spike is different depending on which odorant it is. This happens because the cells have a higher affinity to the first odorant and, consequently, we need a smaller concentration for making them spike. ~ 37 ~ A compartmental model of an olfactory receptor neuron Figure 5.11: Firing rate of 14 cells depending on the stimulus. Showing the response after applying two different odorants. The affinity of the cells to the first odorant is 10-6 and to the second one is 10-2. Note that the amount of odorant here is not expressed as factor of KD, but it is the real value in 10base logarithm. ~ 38 ~ A compartmental model of an olfactory receptor neuron 5.4. Bursting In some extreme experiments (with high amounts of stimulus, high values of gor and/or high values of gKCa) we realized that the cell model starts to burst. Bursting is a dynamic state where a neuron repeatedly fires discrete groups of spikes. Each group is followed by a period of inactivity before the next burst occurs. Bursting is important for the control of the communication and synchronization between neurons. It is known that nearly all kinds of cells can burst when they are driven by a constant, subthreshold input, but in our case the bursting was intrinsic, produced by the calcium current. Even if we were not looking for this behavior, there exist studies that confirm that bursting is intrinsic to olfactory receptor cells [Bobkov and Ache, 2007]. An increase of the stimulus intensity leads to a higher number of spikes per burst, but it does not make the frequency increment sharply because the number of bursts per second slightly decreases. Figure 5.12 and Figure 5.13 show the bursting response of one cell to two different concentration of stimulus. In Figure 5.12 the response is organized in doublets (two-spikes bursts) but incrementing the amount of stimulus, in Figure 5.13, the response is organized in triplets. ~ 39 ~ A compartmental model of an olfactory receptor neuron Figure 5.12: Bursting spiking response organized in doublets. gor=0.00025, gKCa=0.01, amount=0.25 (KD*100.25) ~ 40 ~ Figure 5.13: Bursting spiking response organized in triplets. gor=0.00025, gKCa=0.01, amount=0.75 (KD*100.75) A compartmental model of an olfactory receptor neuron 5.5. Biologically implausible behavior When the amounts of concentration of the input or the gor or gKCa values were even higher, normally we got more non-desired behaviors, as we can see in Figure 5.14 and Figure 5.15. A reason for this biologically implausible behavior might be the calcium current, and probably it could be fixed by exchanging it with a non-bursting calcium current. This was not further regarded, because we were interested in the qualitative behavior in a biologically realistic manner. Figure 5.14: Response of a cell with high gor to different amounts of an odorant. Increasing the amount left to right and top to bottom. gor=0.0064 and gKCa=0.075. The number overprinted on the images indicate the amount of stimulus applied to each experiment (expressed, as usually, in factor ~ 41 ~ of KD in 10-base logarithm) A compartmental model of an olfactory receptor neuron In Figure 5.14 we can see the evolution of the behavior of a cell with high gor when we increase the amount of odorant applied to it. With small amounts we can see that the response behave as we could expect, with high enough spikes that increase their frequency when the concentration in the input increased. With amount -1 (this is KD*101 , 5th picture) the first bursting response occurs, with some doublets, and the height of the spikes starts to decrease. When we increase the amount even more, the spiking response gets this strange shape during the first milliseconds which, at the beginning, we attributed to numerical problems, but after investigating we found out that it is a kind of bursting. Anyway, these results were not useful for our purposes. At very high concentration the cell keeps reacting in similar way and we can even see how the frequency slightly decreases (last image). Figure 5.15: Response of a cell with high gor. The values are: amount=-1 (KD*10-1), gKCa=0.05 and gor=0.01 ~ 42 ~ A compartmental model of an olfactory receptor neuron 5.6. Time varying input In some of the experiments, when the stimulus was too strong, we experienced numerical problems at the initial state, because of the instantaneous increase of stimulus, which leads to a too high frequency. The neuron was receiving the entire stimulus at the same time, as a square pulse (Figure 5.16) but, in a real system, breathing modulates the input stimulus. For solving this problem we decided to apply a sigmoidal function at the beginning of the input. In this way the stimulus arrives to the cell more slowly (see Figure 5.17). Figure 5.16: On the top, spiking response when the cell receives the entire stimulus at the same time. On the bottom, curve showing how the input is applied ~ 43 ~ A compartmental model of an olfactory receptor neuron Figure 5.17: On the top, spiking response when the entry of the input is done slowly, following a sigmoide function. On the bottom, curve showing how the input is applied ~ 44 ~ A compartmental model of an olfactory receptor neuron In Figure 5.18 we can see the simulation of the breathing of a rat, with a breathing period of 200 ms. The odor that the cell receives is always the same, that is why we can see some adaptation, after the first periods. Figure 5.18: On the top, spiking response when the cell receives the input simulating rat breathing. On the bottom, curve showing how the input is applied ~ 45 ~ A compartmental model of an olfactory receptor neuron In the following experiment several different cells were stimulated with time-varying input stimulus during ten seconds. The input will change in time, being three different odors, each one will be applied to the cells during 320 ms, as shown in Figure 5.19. We can see that the first odorant is a mixture of three different odorant and the second and third are pure odorants in a higher concentration. Figure 5.19: Curve showing how the input is applied. In the first odor, each odorant is applied in a concentration of 10-7.7 while in the second and the third odor the concentration of odorant is 10-7 We chose the most descriptive responses after applying the described input to several neurons (Figure 5.20) to compare different behaviors. The cells of the two curves on the top of Figure 5.20 share the same KD values, but the gor for the first one is 0.0256 while for the second one is 0.0064. We can see that they react at the same odorants but with different intensities. The lower left curve shows the response of a cell with small gor, 0.0004. Still, the affinity to the first odor is high enough to produce some spikes. The lower right curve has the same gor value that the upper right one, that is 0.0064, but has different KD values. We can see that, for the first odor, the cell of the upper right experiment is more sensitive than the lower right one. However, it is the opposite for the second odor, because the lower right neuron spikes more. ~ 46 ~ A compartmental model of an olfactory receptor neuron Figure 5.20: Responses of different cells to an input of three consecutive odors ~ 47 ~ A compartmental model of an olfactory receptor neuron 5.7. Visualization Figure 5.21 shows a screenshot of the visualization program that I implemented with OpenGL. The program displays a surface with four hundred cells that simulates a simplified epithelium with four hundred olfactory receptor neurons. They are distributed in ten groups of forty cells, each group occupies two rows (the rows are seen from bottom to left) and correspond to different kinds of neurons. ORNs from the same type share the same KD values (see section 3.2.1) but all cells have a random gor in the range [0.0001 - 0.4096]. When a cell is spiking it is colored in purple (darker) otherwise the cell keep green color (lighter). In this experiment the seventh group of neurons (occupying the thirteenth and fourteenth row) has a very small affinity to the odorant applied as stimulus, and as one can see in the picture none of the cells of this group is spiking. Figure 5.21: Screenshot of the visualization program. The purple (darker) cells correspond to spiking ORNs. ~ 48 ~ A compartmental model of an olfactory receptor neuron 6. Discussion and future work I present here the discussion about the analysis of the results and conclude the report with suggestions and possible ideas for next steps. 6.1. Discussion As the analyses of the results have already been done in the Results section, here I will only do a brief outline of the conclusions that can be drawn comparing our results with the existing data in the literature. Comparing the results of our experiments with the available data in the paper that we took as a reference [Rospars et al., 2003], we conclude: As we can see in section Firing threshold, Fig. 3 of Rospars' paper, the range that firing thresholds in their experiments cover is between 10-3 and 10-10 M. In our model we can achieve an even more wide range, modifying the values of different parameters such as the affinity, the gor or the gKCa. However, as we can see in section 5.3, Figure 5.11 of this thesis with concrete values for this parameters, the same range of thresholds can be achieved. Figures about the latencies look also quite similar. As it is written in Rospars paper, “Fitting of individual latencies in a record set an exponential function with a negative exponent and a nonzero horizontal asymptote gave the best overall results”. Even if we have not done an exponential fit, the apparency of the curves in Fig. 4 in Rospars' is very similar to our curves (section 5.1, Figure 5.2 and Figure 5.4). It is true that, as we can see in Fig. 5, in Rospars' paper, the latency in their experiments is much larger than ours (Figure 5.4). Our latencies fluctuate between 35 and 5 ms, while in their experiments the latency is around 1 s. However, we have to point out that their experiments have been applied to living cells, where the odorants have to travel through the olfactory mucous covering the ORN cilia, before they reach the olfactory receptor. Apart from that, it is likely that the biochemical network of their investigated ORN lead to delays that we are not taking into account in our model. Finally, as we can read in Frequencies section (page 1143 in Rospars' paper), most of their response frequencies were “monotonously increasing and of sigmoid shape”, and we can see this behavior in Fig. 9b of the same paper. Again, this agrees with the responses gotten by us, as we can see in Figure 5.8 or Figure 5.9. This alignment with experimental data is good for some of the simulation experiments (probably the ones with more realistic parameters), after exceeding thresholds of some variables (e.g. applying too high amounts of stimulus, or setting cell parameters to too high values), the behavior changes (see Biologically implausible behavior in section 5.5), and we could not find an obvious explanation to this behavior. In the paper by Izhikevich [Izhikevich, 2000] we found similar behaviors on some of their models. Figure 66 and Figure 69 of the mentioned paper show some bursting ~ 49 ~ A compartmental model of an olfactory receptor neuron responses quite similar to the ones shown in Figure 5.14 and Figure 5.15 in this paper. However, we can not assure that there is any relation between the pictures (a part of a reasonable likeness) as Izhikevich presumably starts from very different assumtions: integrate-and-fire neurons which are not of HH type (i.e. no distinct ion channels). 6.2. Future work After the conclusions, we see that our model reasonably well replicates ORN behavior in a limited range of realistic situations, but one of the main things that we should improve of our model is the behavior in extreme situations. This behavior should be studied in detail, because, even if it was out of our understandability maybe it was not biologically implausible, and the results of the experiments could have been taken into account. As we assume that this behavior occurs because of the bursting calcium current, an obvious solution would be change the calcium current and make it nonbursting current. In that way, we could repeat the experiments and get results in a larger range of values (for both, cells parameters and amount of input). Also simulating the diffusion in the mucous layer could change the dynamics quite a lot. It would be interesting to compare the response of our OE model with the ORN population model [Sandström et al., 2009]. On the other hand, to accomplish the purpose of using our ORN model as input for the already built olfactory bulb model done by MSc Malin Sandström, it is necessary: First, to increase the number of cells. The actual amount of cells, runnable in an average personal computer, is not enough for simulating a real olfactory epithelium (with millions of ORN). For doing it, it is necessary to tune the model to run on a cluster computer (e.g. Blue Gene/L) and set up an array of ORNs of different types. The fact that the code is written in NEURON language will make this task easier, as NEURON allow implementing easily large network models for being run in parallel. The second step is to connect the output of the olfactory epithelium model to provide input to the olfactory bulb model. This could be done via the MUSIC interface. Nowadays, MSc Bernhard Kaplan is working on this as part of his PhD project. Once this additional work is done, it is necessary to analyze the output of the OB model for different stimuli of pure odorants and mixtures. Finally, to achieve a complete olfactory system, it is necessary to design and implement an olfactory cortex which will receive as an input the output from the olfactory bulb, as in a real olfactory system. This work is also currently being done, in the research group of Professor Anders Lansner. ~ 50 ~ A compartmental model of an olfactory receptor neuron References Rospars J. P., Lánský P., Duchamp A. and Duchamp-Viret, P., Relation between stimulus and response in frog olfactory receptor neurons in vivo. European Journal of Neuroscince, 2003; 18(5):1135-1154 Pospischil M., Toledo-Rodriguez M., Monier C., Piwkowska Z., Bal T., Frégnac Y., Markram H. and Destexhe A., Minimal Hodgkin-Huxley type models for different classes of cortical and thalamic neurons. Biological Cybernetics, 2008; 99:427-41 Carnevale N. T. and Hines M. L., The NEURON book. Cambridge University Press, 2006 Kernighan B. W. and Pike R, The Unix Programming Environment. Prentice-Hall Software Series, 1984 Sandström M., Lansner A., Hellgren-Kotaleski J. and Rospars J. P., Modeling the response of a population of olfactory receptor neurons to an odorant. Journal of Computational Neuroscience, 2009; 27(3):337-55 Wachowiak M. and Cohen L. B., Representation of Odorants by Receptor Neuron Input to the Mouse Olfactory Bulb. Neuron, 2001; 32(4):723-35 Sandström M., Computational Modelling of Early Olfactory Processing, PhD Thesis at KTH, 2010 Izhikevich E.M., Neural excitability, spiking and bursting. International Journal of Bifurcation and Chaos, 2000; 10(6):1171–1266 Bobkov Y.V. and Ache B.W., Intrinsically bursting olfactory receptor neurons. Journal of Neurophysiology, 2007; 97:1052-1057 Moczydlowski E. and Latorre R., Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. The Journal of General Physiology, 1983; 82:511-42 Destexhe A., Neubig M., Ulrich D. and Huguenard J., Dendritic low-threshold calcium currents in thalamic relay cells. The journal of Neuroscience, 1998; 18:3574-88 Schild D. and Restrepo D., Transduction mechanisms in vertebrate olfactory receptor cells. Physiological Reviews, 1998; 78:429-466 Buck L. and Axel R., A novel multigene family may encode odorant receptors: a molecular basis for the odor recognition. Cell, 1991; 65(1):175-87 ~ 51 ~ A compartmental model of an olfactory receptor neuron Mombaerts P., Molecular Biology of Odorant Receptors in Vertebrates. Annual Review of Neuroscience, 1999; 22:487-509 Lodish H. F., Berk A. and Kaiser C. A., Molecular cell biology. WH Freeman, 2008 Bibliography Shepherd G. M., The Synaptic Organization of the Brain. Oxford University Press, 2004 Shin J., Adaptation in spiking neurons based on the noise shaping neural coding hypothesis. Elsevier, 2001; 907-919(13) Koch C. and Segev I., Methods in Neuronal Modeling. The MIT Press, 1998 ~ 52 ~ TRITA-CSC-E 2010:050 ISRN-KTH/CSC/E--10/050--SE ISSN-1653-5715 www.kth.se