Part I Atomic Emission Spectrum Lab and Pre
advertisement

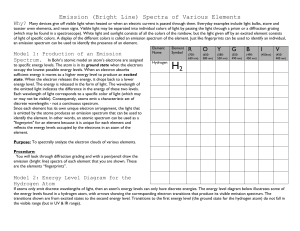
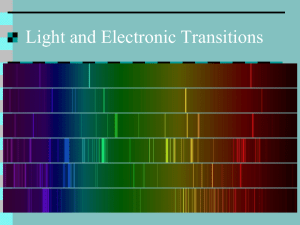
Name_________________________Per.___ Date_____________ Part I of the Atomic Emission Spectrum Lab READ and highlight In the seventeenth century, Sir Isaac Newton discovered that the light of the Sun could be separated into a rainbow of colors using a special piece of glass called a prism. This rainbow of color is called a spectrum. Newton's experiment showed that even though the Sun appears yellowish-white when you look at it (but don't do that often!), the light of the Sun contains all colors of light, from red to violet. Use a piece of spectrum paper and or a hand held spectroscope and hold it up to the light and see ROY G BIV. In the middle of the nineteenth century, it became apparent to scientists that each element had its own pattern of spectrum lines. These spectrum lines could be used, like a fingerprint, to identify elements. Spectrum lines are created by processes that occur at the atomic level. To understand these processes, we must first understand the atom itself. An atom consists of a nucleus containing protons and neutrons. The electrons are found outside the nucleus. The electrons are always moving outside the nucleus within orbitals. An electron can be in orbital #1 or orbital #2, but it can't be halfway in-between them. Since these orbitals are so specific, it takes a very precise amount of energy to move an electron from one orbital to another. Therefore, an electron will only absorb certain photons, those with exactly the right amount of energy to move the electron between orbitals. Remember, the energy of a photon is related to its wavelength, which is related to its color. Once the electrons have been moved to a higher orbital, they are called excited. An excited atom can give back up the absorbed photon, re-emitting it. This re-emission of photons is what causes emission lines. In this experiment you will be looking at the Emission Spectra of several different elements. These elements are present in sealed glass tubes which contain a pure gas of the given element. The tubes are placed in a special holder that passes an electrical current from one end of the tube to the other, like in a neon light. The electricity passing through the gas excites the electrons in the gas molecules and promotes them to higher energy levels. When they return to lower energy states, they emit characteristic wavelengths of light, an emission spectrum. Within every atom there are many different electronic transitions possible. Therefore, there are many different wavelengths of light emitted. In order to separate the different wavelengths of light you will use a hand held spectroscope or spectrum paper. These spectroscopes and spectrum paper separate light into its component wavelengths. The result is the Emission Spectrum of that element. You are required to make some observations using the hand held spectroscope and or spectrum paper for each sample of gas tested. Record your findings below. Using the data you recorded on the known gases you should be able to identify two unknown samples. You will do this by finding the element that has all or most of the lines that you recorded. No two elements should have all the same lines, so you will be able to identify an unknown. Name of the Element Hydrogen Neon Mercury Helium Nitrogen Air Colors observed, wavelengths Identity Unknown #1: Identity Unknown #2: Colors observed: Colors observed: Research how the Northern lights are produced. Find other applications of the line emission spectrum. Pre-lab for Part II Atomic Emission Spectrum Lab Use the following links on the CH5 webpage: Spectral Lines Quantum Theory Niels Bohr Model of the atom Schrodinger's Atom Different Models of the Atom Use complete sentences. 1. How do you excite electrons? 2. What happens to an excited electron? 3. What is the difference between ground state and excited state? 4. What subatomic particle found in an atom is responsible for the release of light? What are those particles doing when the light is released? 5. Why do you think the chemicals have to be heated in the flame first before the colored light is emitted? 6. What is an atomic fingerprint? 7. Why do different chemicals emit different colors of light? 8. What is the characteristic flame color for Sodium, Lithium, Barium, Copper, Potassium and Calcium? 9. Draw a picture of a sodium atom (atomic level) and illustrate how a line spectrum is produced in your drawing. Draw the nucleus and subatomic particles, and the energy levels with electrons. Label your drawing: ground state, excited state, endothermic, exothermic, valence electrons.