LAB #2 - Biology Lab Skills
advertisement

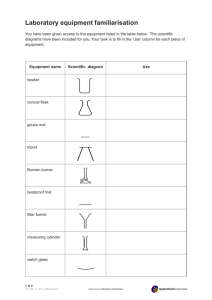
Name: ______________________________ AP Biology – Lab 02 LAB 02 – Biology Lab Skills Objectives: To understand the importance of laboratory safety. To understand how liquid volumes are measured. To investigate the accuracy of beakers, graduated cylinders, Erlenmeyer flasks, volumetric flasks, pipettes, and micropipettes. To demonstrate repeatable, accurate pipetting techniques. To learn how to utilize Excel for organizing statistics and creating graphs. To understand the use of inferential statistics in experiments (includes mean, standard deviation, standard error, probability, [Chi-square] analysis). To learn the basics of spectrophotometry and colorimetry. To learn how to use a spectrophotometer. Introduction: Welcome to an advanced college biology class. You might not really understand what you are in for. When you were just a wee freshmen taking Living Environment, you might have thought that all biology was like that. However, it is not. There is a lot more to biology than what you saw from that class. The exercises in this ―Lab‖ have the purpose of getting you familiar with some of the basic skills that you will use throughout the year and then beyond. Lab Safety: The following safety rules apply to all laboratory areas. Do not eat or drink in the laboratory, unless so directed by the instructor. Wear safety glasses or goggles when directed by your instructor, or during exercises in which glassware and solutions are heated, or during exercises in which dangerous fumes may be present, creating a possible hazard to eyes or contact lenses. Clothes should be appropriate for the laboratory setting… Wear closed-toe shoes at all times in the laboratory. Assume that all reagents are poisonous and act accordingly. Read the labels on reagent containers for safety precautions, and understand the nature of the chemical that you are using. Stopper or cap all reagent bottles when not in use. If reagents come into contact with your skin or eyes, wash the area immediately with water and immediately inform the laboratory instructor. Do not pipette anything by mouth. Discard chemicals as instructed; if you have a question, ask your instructor. Do not pour chemicals back into containers. Do not taste or ingest any reagents. Exercise great caution when using heat, especially when heating chemicals. Do not leave a Bunsen burner or other flame source unattended. Do not light a Bunsen burner near flammable materials. Do not move a lit Bunsen burner. Keep long hair and loose clothing restricted and well away from any flame. Turn the gas valve off when the Bunsen burner is not in use. Use proper ventilation and hoods when instructed. Do not leave stopper in place when heating test tubes. Handle hot glassware or lab ware with a test tube clamp or tongs. Page 1 of 40 Name: ______________________________ AP Biology – Lab 02 Do no operate any laboratory equipment until you have been instructed in its use. Do not perform unauthorized experiments. Keep your work area neat, clean, and organized. Read and understand the experiments you will be doing before coming to the laboratory. Follow the procedures set forth by the laboratory manual. Know the location of emergency equipment: first aid kit eyewash bottle fire extinguisher showers fire blanket Report all accidents to your laboratory instructor immediately. Discard cracked or broken glass only in the broken glass containers. Report all unsafe conditions to your instructor. Clean your work area and glassware and wash your hands before leaving the laboratory. PART I – Measurement, Accuracy, Precision, and Graphical Analysis Measurement plays a particularly large role in science. In their studies, scientists gather data, and to do this they use measurements. Scientists measure the concentration of gases in the atmosphere, the growth of organisms under varying conditions, the rate of biochemical reactions, the distance of stars from the earth, and an innumerable number of other things. As measurements form the basis of scientific inquiry, they are deserving of in-depth analysis in lab. Biology students, as part of their laboratory experience, will be asked to make measurements or observations during the course of their investigations. These may be either qualitative or quantitative. A qualitative measurement/observation describes a characteristic and is not numerical; a quantitative measurement/observation is numerical. Scientific observations are usually made as quantitative as possible so that they are easier to evaluate. For example, if you were trying to find the insect with the world's largest wingspan, quantitative observations are needed. An insect's wingspan may be qualitatively described as huge, but a quantitative description of an insect‘s wing span as 20 centimeters would be much more useful for this project. In a scientific experiment, the investigator examines the effects of variations in the independent variable on the dependent variable through measurements. For example, let's assume a biologist is studying the effect of temperature on plant growth. She sets up several different temperature conditions, and grows groups of plants from seedlings in each condition. When the experiment ends, she must compare plant growth in the plants from different temperatures. But how should she do this? Should she just look at the plants and decide which grew the best? Should she pick up the plants and "feel" which ones have the greatest mass? Of course not… She would use some sort of quantitative measurement, such as measuring the height of each plant's stem in centimeters or determining the total plant biomass in grams. Whichever measurement she chooses, she would need to utilize an instrument to make it. Take a minute and look around you at the variety of objects surrounding you. There are probably a few pens or pencils, a notebook or two, and maybe assorted glassware. While you may be able to easily distinguish the differences between some objects (e.g., your notebook is longer than your pen), other differences are more difficult to discern. You may not be able to easily determine, for example, whether your computer keyboard or your course textbook has greater mass. Even when we can distinguish differences, it is not always easy to determine the Page 2 of 40 Name: ______________________________ AP Biology – Lab 02 extent of those differences. You may have noted that your computer monitor is heavier than your notebook, but is it twice as heavy or three times as heavy? Using only our senses, we cannot be certain about the answer, so we must take measurements. To take measurements we need instruments. Instruments include simple things like rulers and graduated cylinders, and complicated electronics like pH sensors and mass spectrometers. All of these instruments provide us what our senses cannot – a quantified measure of the properties of an object. As all scientific measurements utilize the metric system, we must first say a few things about it before proceeding on. The metric system is universally used in science because it is a decimal system of measurement and is easy to convert from one unit of related measurement to another (e.g ., volume to mass). The metric reference units are the meter (m) for length, the liter (l) for volume, and the gram (g) for mass. Prefixes are used as part of a unit to indicate a portion of or a multiple of a reference unit. For example, the prefix milli (m) indicates .001 (1/1000). A millimeter (mm) is 0.001 (1/1000) of a meter. A milliliter (ml) is 0.001 (1/1000) of a liter. A milligram (mg) is 0.001 (1/1000) of a gram. Note that the same prefixes are used throughout the metric system, regardless of whether we‘re discussing length, volume or mass. See Appendix A in your binder to see many of the metric prefixes that we will be using this year. Volumetrics Typical volumetric containers used in biology labs include beakers, flasks, cylinders, and pipettes. In most cases, these are made of glass and calibrated at a specific temperature. However, for very small volume measurements, micropipettes, constructed out of plastic, with disposable tips, are used. A beaker is a simple container for liquids, very commonly used in laboratories. Beakers are generally cylindrical in shape, with a flat bottom. Beakers are available in a wide range of sizes, from 10 mL up to several liters. They may be made of glass (typically Pyrex®) or of plastic. Beakers may be covered, perhaps by a watch glass, to prevent contamination or loss of the contents. Beakers are often graduated, or marked on the side with lines indicating the volume contained. For instance, a 250 mL beaker might be marked with lines to indicate 50, 100, 150, 200, and 250 mL volumes. The accuracy of these marks can vary from one beaker to another. A beaker is distinguished from a flask by having sides which are vertical rather than sloping. In the lab, beakers are used more often than flasks. A graduated cylinder is used to accurately measure out volumes of liquid chemicals. They are more accurate and precise for this purpose than beakers or flasks. Often, the largest graduated cylinders are made of polyethylene or other rigid plastic, making them lighter and less fragile than glass. Laboratory flasks come in a number of shapes and a wide range of sizes, but a common distinguishing aspect is a wider vessel "body" and one (or sometimes more) narrower tubular sections at the top called necks which have an opening at the top. Like beakers, laboratory flask sizes are specified by the volume they can hold. Laboratory flasks have traditionally been made of glass, but can also be made of plastic. Volumetric flasks come with a stopper or cap for capping the opening at the top of the neck. Stoppers can be made of glass, plastic, or rubber. In general, flasks can be used for making, holding, containing, collecting, or volumetrically measuring solutions. Page 3 of 40 Name: ______________________________ AP Biology – Lab 02 A pipette is a laboratory instrument used to transport an accurately measured volume of liquid. Pipettes are commonly used in biology. Pipettes come in several designs for various purposes with differing levels of accuracy and precision, from single piece flexible plastic transfer pipettes to more complex adjustable or electronic pipettes. A pipette works by creating a vacuum above the liquid-holding chamber and selectively altering this vacuum to draw up and dispense liquid. Pipettes that dispense between 1 and 1000 L (1 mL) are termed micropipettes, while macropipettes (or regular pipetets) dispense greater volumes of liquid. Note, that volumetric pipettes are designed in such a way that after a fluid is dispensed, a small drop of liquid will remain in the tip. In general you should not blow this drop out. The correct volume will be dispensed from the pipette if the side of the tip is touched to the inside wall of the flask (or beaker). Some types of the volumetric glass can be used only to measure predefined volume of solution. Volumetric flasks are designed to contain (TC, sometimes marked as IN) known volume of the solution, while pipettes are generally designed to deliver (TD, sometimes marked as EX) known volume (although in some rare cases they can be designed to contain). This is an important distinction – when you empty a pipette to deliver exactly required volume and you don‘t have to worry about the solution that is left on the pipette walls and in pipette tip. At the same time you will never know how much solution was in the pipette. On the contrary, volumetric flask is known to contain required volume, but if you will pour part the solution to some other flask you will never know how much of the solution was transferred. Both kinds of glass were designed this way as they serve different purposes. Volumetric flask is used to dilute original sample to known volume, so it is paramount that it contains exact volume. Pipette is used to transfer the solution, so it is important that it delivers known volume. Page 4 of 40 Name: ______________________________ AP Biology – Lab 02 Procedure 1: The accuracy of 100 mL beakers 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 100 mL beaker, as carefully as possible, measure 20 mL of de-ionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. Flick any remaining water in the 100 ml beaker into the 250 mL beaker. 4. Record the total mass of the water (from this step) to the nearest 0.01 g in Table 1. 5. Again, using the 100 mL beaker, measure 20 mL of water and add it to the 250 mL beaker. 6. Record the new mass in Table 1. 7. Repeat this procedure until you have 100 mL of water in the beaker on the balance. 8. Calculate the error columns in Table 1. Table 1: Mass of water volumes measured using a 100 mL beaker. Total volume of water (mL) Total expected Total measured Expected mass Measured mass mass of water mass of water of water added of water added (g) (g) in this step (g) in this step (g) 20.0 20.00 40.0 40.00 60.0 60.00 80.0 80.00 100.0 100.00 + / – Error (g) Percent Error Mean Values: Procedure 2: The accuracy of 100 mL graduated cylinders 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 100 mL graduated cylinder, as carefully as possible, measure 20 mL of deionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 2. 5. Again, using the 100 mL graduated cylinder, measure 20 mL of water and add it to the 250 mL beaker. 6. Record the new mass in Table 2. 7. Repeat this procedure until you have ~100 mL of water in the beaker on the balance. 8. Calculate the error columns in Table 2. Page 5 of 40 Name: ______________________________ AP Biology – Lab 02 Table 2: Mass of water volumes measured using a 100 mL graduated cylinder. Total volume of water (mL) Total expected Total measured Expected mass Measured mass mass of water mass of water of water added of water added (g) (g) in this step (g) in this step (g) 20.0 20.0 40.0 40.0 60.0 60.0 80.0 80.0 100.0 100.0 + / – Error (g) Percent Error Mean Values: Procedure 3: The accuracy of 50 mL graduated cylinders 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 50 mL graduated cylinder, as carefully as possible, measure 20 mL of de-ionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 3. 5. Again, using the 50 mL graduated cylinder, measure 20 mL of water and add it to the 250 mL beaker. 6. Record the new mass in Table 3. 7. Repeat this procedure until you have 100 mL of water in the beaker on the balance. 8. Calculate the error columns in Table 3. Table 3: Mass of water volumes measured using a 50 mL graduated cylinder. Total volume of water (mL) Total expected Total measured Expected mass Measured mass mass of water mass of water of water added of water added (g) (g) in this step (g) in this step (g) 20.0 20.0 40.0 40.0 60.0 60.0 80.0 80.0 100.0 100.0 Mean Values: Page 6 of 40 + / – Error (g) Percent Error Name: ______________________________ AP Biology – Lab 02 Procedure 4: The accuracy of 25 mL graduated cylinders 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 25 mL graduated cylinder, as carefully as possible, measure 20 mL of de-ionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 4. 5. Again, using the 25 mL graduated cylinder, measure 20 mL of water and add it to the 250 mL beaker. 6. Record the new mass in Table 4. 7. Repeat this procedure until you have 100 mL of water in the beaker on the balance. 8. Calculate the error columns in Table 4. Table 4: Mass of water volumes measured using a 25 mL graduated cylinder. Total volume of water (mL) Total expected Total measured Expected mass Measured mass mass of water mass of water of water added of water added (g) (g) in this step (g) in this step (g) 20.0 20.0 40.0 40.0 60.0 60.0 80.0 80.0 100.0 100.0 + / – Error (g) Percent Error Mean Values: Procedure 5: The accuracy of 25 mL Erlenmeyer flask 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 25 mL Erlenmeyer flask, as carefully as possible, measure 20 mL of de-ionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 5. 5. Again, using the 25 mL Erlenmeyer flask, measure 20 mL of water and add it to the 250 mL beaker. 6. Record the new mass in Table 5. 7. Repeat this procedure until you have 100 mL of water in the beaker on the balance. 8. Calculate the error columns in Table 5. Page 7 of 40 Name: ______________________________ AP Biology – Lab 02 Table 5: Mass of water volumes measured using a 25 mL Erlenmeyer flask. Total volume of water (mL) Total expected Total measured Expected mass Measured mass mass of water mass of water of water added of water added (g) (g) in this step (g) in this step (g) 20.0 20.0 40.0 40.0 60.0 60.0 80.0 80.0 100.0 100.0 + / – Error (g) Percent Error Mean Values: Procedure 6: The accuracy of 100 mL volumetric flask 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 100 mL volumetric flask, as carefully as possible, measure 100 mL of de-ionized (DI) water. 3. Pour this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 6. 5. Pour the contents in the 250 mL beaker down the sink, dry completely, and return to the top-loading balance. Tare the balance. 6. Again, using the 100 mL volumetric flask, measure 100 mL of water and add it to the 250 mL beaker. 7. Record the mass in Table 6. 8. Repeat steps 5 – 7 for a total of 5 separate, independent measurements. 9. Calculate the error columns in Table 6. Table 6: Mass of water volumes measured using a 100 mL volumetric flask. Volume of water (mL) Expected mass Measured mass of water added of water added in this step (g) in this step (g) 100.0 100.0 100.0 100.0 100.0 Mean Values: Page 8 of 40 + / – Error (g) Percent Error Name: ______________________________ AP Biology – Lab 02 Micropipeting and Microquantity Measurement This part of the lab introduces sterile pipeting and micropipeting techniques used often in molecular and microbiology protocols. Mastery of these techniques will be important for good results in these applications. Most microchemical protocols involve very small volumes of DNA and other reagents. These require you to use an adjustable micropipette that measures as little as one microliter (L) a millionth of a liter, compared to millileters (mL) which are only one thousandth of a liter. Figure 1: Diagram of a pipette bulb. Using a Glass Pipette Take a 10 mL glass (or nalgene) pipette. Carefully place the attachment of the three-way bulb (Figure 1) over the mouth of the pipette. Squeeze the air valve (A) and the bulb simultaneously to empty the bulb of air. Place the tip of the pipette below the solution's surface in the beaker. Gradually squeeze the suction valve (S) to draw liquid into the pipette. When the liquid is above the specified volume, stop squeezing the suction valve (S). Do not remove the bulb from the pipette. DO NOT ALLOW LIQUID TO ENTER THE PIPETTE BULB. If the level of the solution is not high enough, squeeze the air valve (A) and the bulb again to expel the air from the bulb. Draw up more liquid by squeezing the suction valve (S). If the level of the solution is above the specified volume (or 0.00 mL in a TD pipette), gently squeeze the empty valve (E) so the meniscus is at the correct mark. Touch the tip of the pipette to the inside of the beaker to remove the drop hanging from the tip. If this drop is not eliminated, the volume transferred will be slightly higher than the volume desired. To transfer the solution into the desired vessel, press the empty valve (E) until the meniscus is at the mark corresponding to the appropriate volume. Touch the tip of the pipette to the wall of the receiving vessel to remove any liquid from the outside of the tip. Record the final volume in the pipette. The volume transferred is equal to the final pipette reading minus the initial pipette reading. Procedure 7: The accuracy of glass pipette measurements 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a 10 mL glass pipette and pipette bulb, as carefully as possible, measure 10 mL of de-ionized (DI) water. 3. Dispense this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 7. 5. Tare the balance. 6. Again, using the 10 mL glass pipette, measure 10 mL of water and add it to the 250 mL beaker. 7. Record the mass in Table 7. 8. Repeat this procedure until you have 50 mL of water in the beaker on the balance. 9. Calculate the error columns in Table 7. Page 9 of 40 Name: ______________________________ AP Biology – Lab 02 Table 7: Mass of water volumes measured using a 10 mL glass pipette. Volume of water (mL) Expected mass Measured mass of water added of water added in this step (g) in this step (g) + / – Error (g) Percent Error 10.0 10.0 10.0 10.0 10.0 Mean Values: Using a Micropipette THE SOLUTIONS FOR THIS PART OF THE LAB ARE COLORED WATER. THE SAFETY PRECAUTIONS LISTED BELOW ARE TO PREVENT DAMAGE TO THE MICROPIPETTE. NEVER NEVER NEVER NEVER SET THE MICROPIPETTE TO A VOLUME BEYOND ITS RANGE. ATTEMPT TO USE THE PIPETTE WITHOUT A TIP IN PLACE. LAY DOWN A PIPETTE THAT HAS A FILLED TIP. LET THE PLUNGER SNAP BACK AFTER WITHDRAWING OR EJECTING FLUID. 1. Take a large-range micropipette (100 L—1000 L). Rotate the control button to the minimum (100) and maximum (1000) values – do not exceed these values! Notice the change in the plunger length as the volume is changed. 2. Push the micropipette end firmly in the proper size tip. 3. While withdrawing or expelling fluid, always hold the vessel at nearly eye-level. It is important that you watch while you pipette. 4. Hold the pipette in a vertical position when filling. 5. To draw fluid, depress the button to the first stop, and hold in this position. Then, dip the tip into the solution to be pipetted, and draw the fluid into the tip by gradually releasing the plunger. 6. Slide the tip out along the inside wall of the reagent tube to dislodge any excess fluid adhering to the outside of the tip. 7. To withdraw the sample, touch the pipette tip to the inside wall of the reaction tube into which you wish to empty the sample. This creates a capillary effect which helps draw fluid out of the tip. 8. Slowly depress the button to the first stop. Then press on to the second stop to blow out the last bit of fluid. Hold the button down in the second position. 9. Slide the pipette out of the reagent tube with the button depressed to the second stop to avoid sucking any liquid back into the tip. 10. To eject the tip, depress the separate thumb button to ‗launch‘ tip into a waste jar. Page 10 of 40 Name: ______________________________ AP Biology – Lab 02 11. To prevent contamination of your reagents: Always add appropriate amounts of a single reagent sequentially to all reaction tubes. Release each reagent drop onto a new location on the inside wall of the reaction tube. In this way you can use the same tip to pipette reagent into each reaction tube. Use a fresh tip for each new reagent you pipette. 12. Label two (2) 1.5 mL tubes and label them A and B. 13. Using the matrix designed for your micropipette, fill each tube to the desire volume. When using a matrix like the one below, to conserve tips, use the same tip for all aliquots of one solution, then discard the tip and go to the next solution. Also be sure to touch a different side of a microtube when you change solutions, so you do not contaminate the tip. Tube Solution I Solution II Solution III Solution IV A 100 L 200 L 150 L 550 L B 150 L 250 L 350 L 250 L 14. Close the tops, and place the reaction tubes in a balanced configuration (see Figure 2 for examples) in the microfuge rotor. Spinning tubes in an unbalanced position will damage the microfuge rotor. Figure 2: Some possible balanced rotor configurations. 15. Spin tubes for a 1-2 second pulse in the microfuge. This will mix and pool reactants into a droplet in the bottom of each tube. 16. You added a total of 1000 L (1 mL) of reactants into each test tube. Now, set your pipette to 1000 L (1 mL), and very carefully withdraw the solution from each tube – there should be no excess fluid in the tube nor any air bubbles in the pipette tip. Discard into the waste beaker. 17. Obtain a mid-range (10 L—100 L) micropipette. 18. Label two (2) 1.5 mL tubes and label them C and D. 19. Using the matrix designed for your micropipette, fill each tube to the desire volume. Tube Solution I Solution II Solution III Solution IV C 15 L 25 L 32 L 28 L D 11 L 44 L 18 L 27 L Page 11 of 40 Name: ______________________________ AP Biology – Lab 02 20. Close the tops, and place the reaction tubes in a balanced configuration in the microfuge rotor. Spinning tubes in an unbalanced position will damage the microfuge rotor. 21. Spin tubes for a 1-2 second pulse in the microfuge. This will mix and pool reactants into a droplet in the bottom of each tube. 22. You added a total of 100 L of reactants into each test tube. Now, set your pipette to 100 L, and very carefully withdraw the solution from each tube – there should be no excess fluid in the tube nor any air bubbles in the pipette tip. Discard into the waste beaker. 23. Obtain a small-range (0.5 L —10 L) micropipette. 24. Label three (3), 1.5 mL reaction tubes and label them E, F, and G. 25. Use the matrix below to add each solution sequentially to each of the three (3) tubes. Be sure to use a fresh pipette tip for each change in solution. Tube Solution I Solution II Solution III Solution IV E 4 L 5 L 1 L ---- F 4 L 5 L ---- 1 L G 4 L 4 L 1 L 1 L 26. Close the tops, and place the reaction tubes in a balanced configuration in the microfuge rotor. Spinning tubes in an unbalanced position will damage the microfuge rotor. See the next page for balanced rotor positions. If you have an odd number of tubes, you can put in blanks that will balance out the arrangement. 27. Spin tubes for a 1-2 second pulse in the microfuge. This will mix and pool reactants into a droplet in the bottom of each tube. 28. You added a total of 10 L of reactants into each test tube. Now, set your pipette to 10 L, and very carefully withdraw the solution from each tube – there should be no excess fluid in the tube nor any air bubbles in the pipette tip. Discard into the waste beaker. Page 12 of 40 Name: ______________________________ AP Biology – Lab 02 Micropipetting Questions Using the diagram below, draw balanced rotor configurations for 5, 7, 8, 9, and 10 tubes. HINT: You must use at least that number of tubes… Which of the following shows an unbalanced rotor? (put an X through the diagram) The small range digital micropipette measures volumes between 0.5 L and 10.0 L. If you wish to dispense seven and five-tenths microliters of a fluid with the instrument, what sequence of numerals would you see on the digital dial? a. 75/00 b. 75/10 c. 00/75 d. 07/50 A student presses the button on the micropipette to the first position, places it in a liquid and slowly releases the button. What will most likely occur? a. ejection of the tip b. fluid will be drawn up into the tip c. the last drop of fluid will be pushed our of the tip d. most, but not all fluid will be expelled from the tip A student presses the button on the micropipette to the second position. What will most likely occur? a. ejection of the tip b. most of the fluid will be removed from the tip c. fluid will be drawn up into tip d. fluid will be ejected and then redrawn into the tip On a large-range Eppendorf digital micropipette, what volume of liquid is indicated by these numbers 0 5 0 0 a. 5 L b. 50 L c. 500 L d. 5000 L Page 13 of 40 Name: ______________________________ AP Biology – Lab 02 Complete the following conversions: a. 0.167 mL to L _________ b. 0.05 mL to L _________ c. 42 L to mL _________ d. 182 L to mL _________ e. 0.9 L to mL _________ Identify four (4) important precautions in micropipette use: _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ Procedure 8: The accuracy of micropipette measurements 1. Using a 400 gram top-loading balance, tare a dry 250 mL beaker. 2. Using a large-range (100 L—1000 L) micropipette with a fresh tip, measure 1000 L (1 mL) of de-ionized (DI) water. 3. Dispense this measured amount of water into the 250 mL beaker. 4. Record the mass of the water to the nearest 0.01 g in Table 8. 5. Tare the balance. 6. Again, using the large range micropipette, measure 1 mL (1000 L) of water and add it to the 250 mL beaker. 7. Record the mass in Table 8. 8. Repeat this procedure until you have 5 mL of water in the beaker on the balance. 9. Calculate the error columns in Table 8. Table 8: Mass of water volumes measured using a large range (100 L—1000 L) micropipette. Volume of water (mL) Expected mass Measured mass of water added of water added in this step (g) in this step (g) 1.000 1.000 1.000 1.000 1.000 Mean Values: Page 14 of 40 + / – Error (g) Percent Error Name: ______________________________ AP Biology – Lab 02 Accuracy and Precision What do these terms mean? Many might think that they are the same. However, there is an important distinction between the two. Accuracy is how close the measured values are to the actual values. Precision is how reproducible or repeatable the measured values are. Measurements can be either accurate, precise, neither, or both. The classic distinction between the two involves shooting arrows at a target. How accurate the archer is can be seen by looking at how close the arrows are to the bulls-eye. The closer to the bulls-eye, the more accurate the archer is. The further from the bulls-eye, the less accurate. How precise the archer is can be shown by how close the ‗cluster of arrows‘ are to each other. The less spread out the arrows are (in relation to each other), the more precise the archer. The more spread out, the less precise. Look at each target below and decide whether the situation is accurate and/or precise. Accurate?: Yes / No Accurate?: Yes / No Accurate?: Yes / No Precise?: Precise?: Precise?: Yes / No Yes / No Yes / No In Procedures 1 – 8, you should have calculated the percent error for each volumetric instrument. The mean of the percent error can be related to the accuracy of each volumetric instrument. Therefore, the lesser the mean is of the percent error, the more accurate the measuring device. Inversely, the greater the mean is of the percent error, the less accurate the measuring device. However, we cannot determine precision from the above. To determine precision, we must look at the variance that exists in the set. We can get this by looking at the standard deviation. The formulas for mean, standard deviation (SD), and standard error of the mean (SEM) are listed below and are also supplied in Appendix A of your binder. SD The standard deviation (SD) describes the variability between individuals in a sample; the standard error of the mean (SEM) describes the uncertainty of how the sample mean represents the overall population mean. Page 15 of 40 Name: ______________________________ AP Biology – Lab 02 If normally distributed (a bell curve – more on that later), the study sample can be described entirely by two parameters: the mean and the standard deviation. The SD represents the variability within the sample; the larger the SD, the higher the variability within the sample. Although the SD and the SEM are related (see formulas), they give two very different types of information. Whereas the SD estimates the variability in the study sample, the SEM estimates the precision and uncertainty of how the study sample represents the underlying population. In other words, the SD tells us the distribution of individual data points around the mean, and the SEM informs us how precise our estimate of the mean is. In Table 9 below, for each volumetric device, fill in your calculated mean, the standard deviation (SD), and the standard error of the mean (SEM) for each set of percent error values. Table 9: Mean, standard deviation, and standard error of the volumetric devices measuring a known quantity of water. Mean percent error (from earlier tables) Volumetric device 100 mL beaker 100 mL graduated cylinder 50 mL graduated cylinder 25 mL graduated cylinder 25 mL Erlenmeyer flask 100 mL volumetric flask 10 mL glass pipette 100 L – 1000 L micropipette Page 16 of 40 Standard deviation Standard Error of the mean Name: ______________________________ AP Biology – Lab 02 Using Excel It is important to not only be able to correctly collect data, but also present it visually – often in chart and graph form. Data tables and visual representations of data are integral parts of the results section of the lab write-ups and mini-posters that you will be producing this year. For general graphing help, refer to Appendix B in your binder. For help with Excel, refer to the summer assignment that was given. If you still need help, make sure you get it – from the teacher or other students in the class. You will have to know how to do this, so make sure you understand the steps to construct a useful graph. Using Excel, create a series of 5 graphs (use scatter plot with trendlines) that show the relationship of total expected mass versus total measured mass of the water samples measured for each volumetric device in Procedures 1 through 5. For each graph, also include a line that shows a direct correlation – you will need to plot a line that is ‗perfect‘ – (0,0), (20,20), (40,40), (60,60), (80,80), (100,100). NOTE: Make sure that you label all axes, complete a reasonable figure caption, have a key/legend of points/lines, etc. These graphs will also be collected. Also using Excel, put all 6 of these lines on one graph (these include the 5 from procedures 1 – 5 and the ‗perfect‘ line) on one grid. Do your best make this multi-line graph easy to interpret – you will need a properly formatted graph with all the bells and whistles (legend, caption, etc.) Compare the lines on the above graph with the mean percent error that you calculated for each volumetric device. Which would you say is the most accurate based on both your calculations from the data table? Which would you say is the most accurate based on the graphical representation of the data? HINT: compare each trendline for the calculated data with the ‗perfectly correlated [R2 = 1.000] line. _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ Page 17 of 40 Name: ______________________________ AP Biology – Lab 02 Again, using Excel, create a bar graph with error bars ( standard error) showing the relationship between type of measuring device and the percentage error. Note: All bars should be positive in this graph. You can find the Error Bars button in Excel 2007 (and I believe it is the same for Office 2010) when you click on the graph and then look under Chart Tools > Layout > Analysis. Compare the bars on the above graph with the mean percent error including the error bars for each volumetric device. Which would you say is the most precise based on both your calculations? Which would you say is the most precise based on the graphical representation of the data? HINT: For the graph, look at the size of the error bars. _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ Page 18 of 40 Name: ______________________________ AP Biology – Lab 02 PART II – Inferential Statistics: Introduction Up to this point, we've discussed the proper methods for taking measurements, and you've gained some experience with simple instruments. In this section, we will follow up on that by introducing a new group of statistics – inferential statistics. Before I define inferential statistics, let me show you why they are useful. Imagine a research study that sought to determine the effects of temperature on plant biomass. Upon completion of an experiment, the experimenters would have sets of biomass measurements for each group. What do they do then? They take the mean of the values for each group and then make conclusions based on this statistic alone? What if the mean biomass for one group is only slightly higher than that for another group – is the difference sufficient for her to make a solid conclusion? Inferential statistics allow you to make comparisons in scientific studies and determine with confidence if differences in treatment groups truly exist. Inferential statistics are used to make comparisons between data sets and infer whether the two data sets are significantly different from one another. It is important to realize that when dealing with statistics and probability, chance always plays a role. When we compare means from two groups in an experiment, we are attempting to determine if the two means truly differ from one another, or if the difference in the means of the groups is simply due to random chance. The best way to explain this concept is with an example… Page 19 of 40 Name: ______________________________ AP Biology – Lab 02 Chance and ―Significant‖ Differences: A Case Study After losing a close game in overtime, a local high school football coach accuses the officials of using a "loaded" coin during the pre-overtime coin toss. He claims that the coin was altered to come up heads when flipped, his opponents knew this, won the coin toss, and consequently won the game on their first possession in overtime. He wants the local high school athletic association to investigate the matter. You are assigned the task of determining if the coach's accusation stands up to scrutiny. Well, you know that a "fair" coin should land on heads 50% of the time, and on tails 50% of the time. So how can you test if the coin in question is doctored? If you flip it ten times and it comes up heads six times, does that validate the accusation? What if it comes up heads seven times? What about eight times? Does coming up heads five time prove that it ISN‘T a rigged coin? To make a conclusion, you need to know the probability of these occurrences. To examine the potential outcomes of coin flipping, we will use a Binomial Distribution. This distribution describes the probabilities for events when you have two possible outcomes (heads or tails) and independent trials (one flip of the coin does not influence the next flip). The distribution for ten flips of a fair coin is shown in Figure 3. Figure 3: Binomial Distribution for fair coin with ten flips. Note that ratio of 5 heads : 5 tails is the most probable, and the probabilities of other combinations decline as you approach greater numbers of heads or tails. The figure demonstrates two important points. One, it shows that the expected outcome is the most probable – in this case a 5 : 5 ratio of heads to tails. Two, it shows that unlikely events can happen due solely to random chance (e.g., getting 0 heads and 10 tails), but that they have a very low probability of occurring. Page 20 of 40 Name: ______________________________ AP Biology – Lab 02 Also note that the binomial distribution is rather "jagged" when only ten coin flips are performed. As the number of trials (coin flips) increases, the shape of the distribution begins to smooth out and resemble a normal curve. Note how the shape of the curve with 50 trials is much smoother than the curve for 10 trials, and more representative of a normal curve as seen in Figure 4. These normal curves are often referred to as a ―bell curve‖. Figure 4: Binomial distribution for fair coin with 50 flips. Inferential Statistics: Probability Normal curves are useful because they allow us to make statistical conclusions about the likelihood of being a certain distance from the center (mean) of the distribution. In a normal distribution, there are probabilities associated with differing distances from the mean. Recall from Algebra 2/Trigonometry that 68% of the values in a sample showing normal distribution are within one standard deviation of the mean, 95% of values are within two standard deviations of the mean, and 99% of the values are within three standard deviations of the mean – Figure 5. Figure 5: Percent of normally distributed values in each interval based on standard deviation. Page 21 of 40 Name: ______________________________ AP Biology – Lab 02 The difficulty with working with probabilities is knowing when to conclude that an occurrence is NOT due to random chance. Values far from the mean in a distribution can occur, but will occur with low probability (Figure 5). We are therefore essentially testing the hypothesis that the observed data fit a particular distribution. In the coin flip example, we're testing to see if our results fit those expected from the distribution of a fair coin. So we need to come up with a point at which we can conclude our results are definitely not part of the distribution we are testing. So when do you determine that a given data set no longer fits a distribution when random chance will always play a role? Well, you've got to make an arbitrary decision, and biologists/statisticians set precedent long ago. Given that 95% of the values in a distribution fall within two standard deviations of the mean, statisticians have decided that if a result falls outside of this range, you can determine that your data does not fit the distribution you are testing. This essentially says that if your result has equal to or less than a 5% chance of belonging to a particular distribution, then you can conclude with 95% confidence/certainty (meaning outside the 2 standard deviation interval) that it is not a part of that distribution. As probabilities are listed as proportions, this means that a result is "statistically significant" if its occurrence (p-value) is equal to or less than 0.05. This leads to our statistical "rule of thumb" - whenever a statistical test returns a probability value (or "p-value") equal to or less than 0.05, we reject the hypothesis that our results fit the distribution we expect to get. The standard practice in such comparisons is to use a null hypothesis (written as "H0"), which states that the data are not statistically significant and do fit the expected distribution along with an alternate hypothesis (written as "HA"), which states that the data are statistically significant and do not fit the expected distribution. H0 : The data fit the assigned distribution with 95% confidence and is not statistically significant. Ha : The data do not fit the assigned distribution with 95% confidence and is statistically significant. To practice your interpretation of p-values, decide if each of the p-values below indicates that you should reject your null hypothesis by circling the correct answer. A. p-value = 0.11 Accept or Reject H0? B. p-value = 0.56 Accept or Reject H0? C. p-value = 0.01 Accept or Reject H0? D. p-value = 0.9 > 0.7 Accept or Reject H0? E. p-value < 0.005 Accept or Reject H0? Page 22 of 40 Name: ______________________________ AP Biology – Lab 02 So back to our coin test… It is comparing our result to the expected distribution of a fair coin. To test the coin, you opt to flip it 50 times, tally the number of heads and tails, and compare your results to the fair coin distribution. Our (complete) null hypothesis would be as follows: H0 : The coin is balanced properly and we would expect an even number of heads and tails when flipped repeatedly with 95% confidence. Ha : The coin is not balanced properly and we would not expect an even number of heads and tails when flipped repeatedly with 95% confidence. You obtain the results listed below: Table 10: Number of times observed when a coin was flipped 50 times. Heads Tails 33 17 So what does this mean? Referencing the distribution (Figure 4 above), we see that a ratio of 33 heads to 17 tails would only occur about 1% of the time if the coin were indeed fair. As this is less than 5% (p < 0.05), we can reject our hypothesis that the data fit the expected distribution. In other words, we reject our null hypothesis with 95% confidence/certainty that the coin was fair and we would expect a 50% heads : 50% tails ratio. We were testing the distribution of a fair coin, so this suggests the coin was not fair, and the coach's accusation has merit. This indicates that further tests should be conducted, and the number of trials (coin flips) increased so a more definitive conclusion could be reached. Man, I love a good controversy... Stating conclusions Once you have collected your data and analyzed them to get your p-value, you are ready to determine whether your original hypothesis is supported or not. If the p-value in your analysis is 0.05 or less (0.05, 0.01, etc.) then the data do not support your null hypothesis with 95% confidence that the observed results would be obtained due to chance alone. So, as a scientist, you would state your "unacceptable" results in this way: "The differences observed in the data were statistically significant at the 0.05 level." You could then add a statement like, "Therefore, with 95% confidence, the data do not support the hypothesis that..." This is how a scientist would state "acceptable" results: "The differences observed in the data were not statistically significant at the 0.05 level." You could then add a statement like, "Therefore, with 95% confidence, the data support the hypothesis that..." And you will see that over and over again in the conclusions of research papers. Page 23 of 40 Name: ______________________________ AP Biology – Lab 02 Chi-Square Analysis The Chi-square is a statistical test that makes a comparison between the data collected in an experiment versus the data you expected to find. It can be used whenever you want to compare the differences between expected results and experimental data. Variability is always present in the real world. If you toss a coin 10 times, you will often get a result different than 5 heads and 5 tails. The Chi-square test is a way to evaluate this variability to get an idea if the difference between real and expected results are due to normal random chance, or if there is some other factor involved (like our unbalanced coin). The Chi-square test helps you to decide if the difference between your observed results and your expected results is probably due to random chance alone, or if there is some other factor influencing the results. In other words, it determines what our p-value is! The Chi-square test will not, in fact, prove or disprove if random chance is the only thing causing observed differences, but it will give an estimate of the likelihood that chance alone is at work. Determining the Chi-square Value Chi-square is calculated based on the formula below. We will fill out a table for the first go around so you can get familiar with how to use it. Follow the following procedure to test the hypothesis that any pair of given coins are evenly balanced. Activity 1. Each team of two students will toss a pair of coins exactly 100 times and record the results in Table 11. The only outcomes can be: both heads (H/H), one heads and one tails (H/T), or both tails (T/T). Based on the laws of probability (that we learned in math years ago), each of these have a 25%, 50%, and 25% chance of happening, respectively. Each team must check their results to be certain that they have exactly 100 tosses. 2. Restate the null and alternate hypotheses for our activity below: H0: _______________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ Ha: _______________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ Page 24 of 40 Name: ______________________________ AP Biology – Lab 02 Table 11: Team data for coin flip test. Toss 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 H/H H/T T/T Toss 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 H/H H/T T/T Toss 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 H/H H/T T/T Total (the sum of the total of each column must equal 100 tosses) 3. Record your team results on the classroom computer and then record the summarized results on the following page in Table 12. Page 25 of 40 Name: ______________________________ AP Biology – Lab 02 Table 12: Class data for our coin flip test. 1 2 3 4 5 6 7 8 9 10 Obs Exp H/H H/T T/T Total 4. Analyze both the team and class data separately (in Tables 13 and 14) using the Chisquare analysis as explained below. A. For your individual team results, complete column A on Table 13 by entering your observed results in the coin toss exercise. B. For your individual team results, complete column B on Table 13 by entering your expected results in the coin toss exercise. In some cases – but not this time – it is okay if you have to use decimals for fractions (½ = 0.5). C. For your individual team results, complete column C on Table 13 by calculating the difference between your observed and expected results. D. For your individual team results, complete column D on Table 13 by calculating the square of the difference between your observed and expected results – this is done to force the result to be a positive number. E. For your individual team results, complete column E on Table 13 by dividing the square in column D by the expected results. F. Calculate the 2 value by summing each of the answers in column E. The means summation. symbol G. Repeat these calculations for the full class data and complete Table 14. H. Enter the ―Degrees of Freedom‖ in Tables 13 and 14 based on the explanation below the data tables. Table 13: Chi-square analysis of individual team data. A B C D E obs exp obs – exp (obs – exp)2 (obs – exp)2 exp H/H H/T T/T 2 total Degrees of Freedom Page 26 of 40 Name: ______________________________ AP Biology – Lab 02 Table 14: Chi-square analysis of class team data. A B C D E obs exp obs – exp (obs – exp)2 (obs – exp)2 exp H/H H/T T/T 2 total Degrees of Freedom Interpreting Chi-Square Value The rows in the Chi-square Distribution table (Table 15) refer to degrees of freedom. The degrees of freedom are calculated as the one less than the number of possible results in your experiment. In the double coin toss exercise, you have 3 possible results: two heads, two tails, or one of each. Therefore, there are two degrees of freedom for this experiment. In a sense, the ―degrees of freedom‖ is measuring how many classes of results can ―freely‖ vary their numbers. In other words, if you have an accurate count of how many 2-heads, and 2-tails tosses were observed, then you already know how many of the 100 tosses ended up as mixed head-tails, so the third measurement provides no additional information. Table 15: The Chi-square distribution table. degrees of freedom probability value (p-value) ACCEPT NULL HYPOTHESIS REJECT 0.99 0.95 0.80 0.70 0.50 0.30 0.20 0.10 0.05 0.01 1 0.001 0.004 0.06 0.15 0.46 1.07 1.64 2.71 3.84 6.64 2 0.02 0.10 0.45 0.71 1.30 2.41 3.22 4.60 5.99 9.21 3 0.12 0.35 1.00 1.42 2.37 3.67 4.64 6.25 7.82 11.34 4 0.30 0.71 1.65 2.20 3.36 4.88 5.99 7.78 9.49 13.28 5 0.55 1.14 2.34 3.00 4.35 6.06 7.29 9.24 11.07 15.09 6 0.87 1.64 3.07 3.38 5.35 7.23 8.56 10.65 12.59 16.81 7 1.24 2.17 3.84 4.67 6.35 8.38 9.80 12.02 14.07 18.48 Page 27 of 40 Name: ______________________________ AP Biology – Lab 02 So which column do we use in the Chi-square Distribution table? The columns in the Chi-square Distribution table with the decimals from 0.99 through 0.50 to 0.01 refer to probability levels of the Chi-square (0.99 = 99%; 0.05 = 5%; etc.). For instance, 3 events were observed in our coin toss exercise, so we already calculated we would use 2 degrees of freedom. If we calculate a Chi-square value of 1.386 from the experiment, then when we look this up on the Chi-square Distribution chart, we find that our Chi-square value places us near the ―p=.50‖ column – in the range of 0.50 > 0.30. This means that the variance between our observed results and our expected results would occur from random chance between 30-50% of the time. Therefore, we could conclude (with 95% confidence – 2 standard deviation interval) that chance alone could cause such a variance often enough that the data still supported our hypothesis, and probably another factor is not influencing our coin toss results. However, if our calculated Chi-square value, yielded a sum of 5.991 or higher, then when we look this up on the Chi-square Distribution chart, we find that our Chi-square value places us beyond the ―p=.05‖ column. This means that the variance between our observed results and our expected results would occur from random chance alone less than 5% of the time (only 1 out of every 20 times). Therefore, we would conclude (with 95% confidence) that chance factors alone are not likely to be the cause of this variance. Some other factor is causing some coin combinations to come up more than would be expected. Maybe our coins are not balanced and are weighted to one side more than another. Variations on the Chi-Square Analysis In medical research, the chi-square test is used in a similar — but interestingly different — way. When a scientist is testing a new drug, the experiment is set up so that the control group receives a placebo and the experimental group receives the new drug. Analysis of the data is trying to see if there is a difference between the two groups. The expected values would be that equal numbers of people get better in the two groups — which would mean that the drug has no effect. If the chi-square test yields a p-value greater than .05, then the scientist would accept the null hypothesis which would mean the drug has no significant effect. The differences between the expected and the observed data could be due to random chance alone. If the chisquare test yields a p-value = .05, then the scientist would reject the null hypothesis which would mean the drug has a significant effect. The differences between the expected and the observed data could not be due to random chance alone and can be assumed to have come from the drug treatment. In fact, chi-square analysis tables can go to much lower p-values than the one above — they could have p-values of .001 (1 in 1000 chance), .0001 (1 in 10,000 chance), and so forth. For example, a p-value of .0001 would mean that there would only be a 1 in 10,000 chance that the differences between the expected and the observed data were due to random chance alone, whereas there is a 99.99% chance that the difference is really caused by the treatment. These results would be considered highly significant. Page 28 of 40 Name: ______________________________ AP Biology – Lab 02 Chi-Square Analysis Questions Based on the laws of probability, what was your hypothesis (expected numbers) for your individual team coin toss? __________________________________________________________________________ __________________________________________________________________________ What was your calculated Chi-square value for your individual team data? _____________ What p-value does this Chi-square value correspond to? Was your hypothesis supported by your results? Explain (completely and correctly) using the Chi-square analysis. _____________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ Based on the laws of probability, what was your hypothesis (expected numbers) for the class coin toss? __________________________________________________________________________ __________________________________________________________________________ What was your calculated Chi-square value for the class data? _____________ What p-value does this Chi-square value correspond to? _____________ Was your hypothesis supported by your results? Explain (completely and correctly) using the Chi-square analysis. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ Page 29 of 40 Name: ______________________________ AP Biology – Lab 02 Significance of a Large Sample Size When designing research studies, scientists purposely choose large sample sizes. Work through these scenarios to understand why… a) Just an in your experiment, you flipped 2 coins, but you only did it 10 times. You collected these data below. Use Table 16 to calculate the Chi-square value. (It is okay to use decimals for your expected column! e.g. [2.5, 5.0, 2.5] – based on percentages) Table 16: Testing coin flipping results with a sample size of 10 flips. obs H/H 1 H/T 8 T/T 1 exp obs – exp (obs – exp)2 (obs – exp)2 exp 2 total Degrees of Freedom Would you accept or reject the preciously stated null hypothesis? Explain (completely and correctly) using the Chi-square analysis guide. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ b) Now you flipped your coins again, but you did it 100 times. You collected these data below. Use Table 17 to calculate the Chi-square value. Table 17: Testing coin flipping results with a sample size of 100 flips. obs H/H 10 H/T 80 T/T 10 exp obs – exp (obs – exp)2 2 total Degrees of Freedom Page 30 of 40 (obs – exp)2 exp Name: ______________________________ AP Biology – Lab 02 Would you accept or reject the null hypothesis? Explain (completely and correctly) using the Chi-square analysis. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ c) Now you flipped your coins again, but you did it 1000 times. You collected these data below. Use Table 18 to calculate the Chi-square value. Table 18: Testing coin flipping results with a sample size of 1000 flips. obs H/H 100 H/T 800 T/T 100 exp obs – exp (obs – exp)2 (obs – exp)2 exp 2 total Degrees of Freedom Would you accept or reject the null hypothesis? Explain (completely and correctly) using the Chi-square analysis. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ d) Explain why scientists purposely choose large sample sizes when they design research studies using data obtained from each of the above analyses. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ Page 31 of 40 Name: ______________________________ AP Biology – Lab 02 PART III – Spectrophotometry Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while light illuminates an object, the color that the eye perceives is determined by the absorption by the object of one or more of the colors from the source of the white light. The remaining wavelength(s) are reflected (or transmitted) as a specific color. Thus an object that appears red absorbs the blue or green colors of light (or both), but not the red. The perception of color, as just described is qualitative. It indicates what is happening but says nothing about the extent to which the event is taking place. The eye is not a quantitative instrument. However, there are instruments, called spectrophotometers, that electronically quantify the amount and kinds of light that are absorbed by molecules in solution. In its simplest form, a spectrophotometer has a source of white light (for visible spectrophotometry— some use UV) that is focused on a prism or diffraction grating to separate the white light into its individual bands of radiant energy. Each wavelength (color) is then selectively focused through a narrow slit. The width of this slit is important to the precision of the measurement; the narrower the slit, the more closely absorption is related to a specific wavelength of light. Conversely, the broader the slit, the more light of different wavelengths passes through, which results in a reduction in the precision of the measurement. This monochromatic (single wavelength) beam of light, called the incident beam (IO), then passes through the sample being measured. The sample, usually dissolved in a suitable solvent, is contained in a an optically selected cuvette, which should be standardized to have a light path 1 cm across. After passing through the sample, the selected wavelength of light (no referred to as the transmitted beam, (I) strikes a photoelectric tube. If the substance in the cuvette has absorbed any of the incident light, the transmitted light will then be reduced in total energy content. If the substance in the sample container does not absorb any of the incident beam, the radiant energy of the transmitted beam will then be about the same amount as that of the incident beam. When the transmitted beam strikes the photoelectric tube, it generates an electric current proportional to the intensity of the light energy striking it. By connecting the photoelectric tube to a device that measures electric current (a galvanometer), a means of directly measuring the intensity of the transmitted beam is achieved. In the spectrophotometers that we will use (called the Spec-20), the galvanometer has two scales: one indicates the % transmittance (%T), and the other, a logarithmic scale with unequal divisions graduated from 0.0 to 2.0, indicates the absorbance (A). Because most biological molecules are dissolved in a solvent before measurement, a source of error can be due to the possibility that the solvent itself absorbs light. To assure that the spectrophotometric measurement will reflect only the light absorption of the molecules being studied, a mechanism for ―subtracting‖ the absorbance of the solvent is necessary. To achieve this, a ―blank‖ (the solvent) is first inserted into the instrument, and the scale is set to read 100% transmittance (or 0.0 absorbance) for the solvent. The ―sample,‖ containing the solute plus the solvent, is then inserted into the instrument. Any reading on the scale that is less than 100% T (or greater than 0.0 A) is considered to be due to absorbance by the solute only. Page 32 of 40 Name: ______________________________ AP Biology – Lab 02 As mentioned earlier, spectrophotometers are not limited to detecting absorption of only visible light. Some also have a source of ultraviolet light (usually supplied by a hydrogen or mercury lamp), which has wavelengths that range from about 180 to 400 nm. Ultraviolet wavelengths ranging from 180 to 350 nm are particularly useful in studying such biological molecules as amino acids, proteins, and nucleic acids because each of these compounds have characteristic absorbances at different UV wavelengths. Other spectrophotometers use infrared radiation (from 780 to 25,000 nm) as well. Units of Measurement The following terminology is commonly used in spectrophotometry. Transmittance (T): the ratio of the transmitted light (I) of the sample to the incident light (IO) on the sample. T= I IO This value is multiplied by 100 to derive the % T. For example: %T= 75 = 100 0.75 Absorbance (A): logarithm to the base 10 of the reciprocal of the transmittance: A = log10 1 T For example: 1. Suppose a % T of 50 was recorded (equivalent to T = 0.50). 2. Then A = log10 (1/0.50) = log10 2.0. 3. Thus log10 2.0 = 0.301 (A equivalent to a % T of 50). 4. Similarly a % T of 25 = 0.602 A; a % T of 75 = 0.125 A; and so forth. The absorbance scale is normally present along with the transmittance scale on spectrophotometers. The chief usefulness of absorbance lies in the fact that it is a logarithmic rather than arithmetic function, allowing the use of the Lambert-Beer law (Beer‘s Law), which states that for a given concentration range the concentration of solute molecules is directly proportional to absorbance. This law can be expressed as log10 IO = A I in which IO is the intensity of the incident light; I is the intensity of the transmitted light. Page 33 of 40 Name: ______________________________ AP Biology – Lab 02 Figure 6: Transmittance and Aborbance of a sample at a given wavelength at various concentrations. The usefulness of absorbance can be seen in the graphs shown in Figure 6, one graph showing the percent transmittance plotted against concentration and the other showing absorbance plotted against concentration. Using the Lambert-Beer relationship, it is necessary to plot only three or four points to obtain the straight-line relationship shown in the bottom graph of the two. However, certain conditions must prevail for the Lambert-Beer relationship to hold: 1. Monochromatic light is used. 2. Amax is used (i.e., the wavelength maximally absorbed by the substance being analyzed). 3. The quantitative relationship between absorbance and concentration can be established. The first condition can be met by using a prism or diffraction grating or other device that can disperse visible light into its spectra. The second condition can be met by determining the absorption spectrum of the compound. This is done by plotting the absorbance of the substance at a number of different wavelengths. The wavelength at which absorbance is greatest is called the Amax (or max) and is the most satisfactory wavelength to use because, on the slope, absorbance changes rapidly with slight wavelength deviations, whereas at the maximum absorbance, changes in wavelength alter absorbance less. Figure 7 shows an absorption spectrum of a hypothetical substance having an Amax of approximately 650 nm. Some compounds, however, can have several peaks both in the visible spectrum and in the ultraviolet range. An example of this is shown in Figure 8 for riboflavin. Figure 7: Absorption spectrum of a known concentration of a sample. Page 34 of 40 Name: ______________________________ AP Biology – Lab 02 Figure 8: Absorption spectrum of riboflavin. To establish the quantitative relationship between absorbance and concentration of the colored substance, it is necessary to prepare a series of standards of the substance analyzed in graded known concentrations (a.k.a. color standards). Because absorbance is directly proportional to concentration, a plot of absorbance versus concentration of the standard yields a straight line. Such a plot is called a standard curve or calibration curve as shown in Figure 9. After several points have been plotted, the intervening points can be extrapolated by connecting the known points with a straight line. It is not necessary to use dotted lines to indicate extrapolation on graphs; a dotted line is used in the illustration to indicate the parts of the line for which points were not determined but were presumed. When the Lambert-Beer law is followed, this is an acceptable and time-saving assumption; otherwise, points would be need to be plotted throughout the entire line. In general, your graph should extend from a minimum of about 0.025 A to a maximum of about 1.0 A, or from 94% T to 10% T, this being the ―readable‖ and reproducible values of absorbance. However, recall that the Lambert-Beer law operates at only certain concentrations. This is apparent in Figure 9, in which, at concentrations greater than 1.0 mg/mL, the curve slopes, indicating the loss of the concentration-absorbance relationship. Figure 9: Absorption curve extended from known values. Page 35 of 40 Name: ______________________________ AP Biology – Lab 02 After a concentration curve for a given substance has been established, it is relatively easy to determine the quantity of that substance in a solution of unknown concentration by determining the absorbance of the unknown an locating it on the y-axis, or ordinate (Figure 10). A straight line is then drawn parallel to the x-axis, or abscissa, until it intersects with the experimental curve. A perpendicular is then dropped to the x-axis, the value at the point of intersection indicating the concentration of the unknown solution. In this example, the unknown absorbance is 0.32, which indicates a concentration of about 0.37 mg. Concentrations are commonly expressed either as micrograms per milliliter (g/mL) or as milligrams per milliliter (mg/mL). Figure 10: Using a standard curve to find the concentration of an unknown sample. If the absorbance value of the unknown is such that the line drawn parallel to the x-axis intersects the experimental curve where it is curved (Figure 9), then you cannot accurately determine the concentration. In this event, dilute the unknown by some factor until the absorbance readings intersect with the straight-line part of the graph where concentration is proportional to absorbance. You can then determine the unknown concentration and multiply the value by the dilution factor. Quantitative Chemical Determination of Protein In protein molecules, the successive amino acid molecules are bonded together between the carboxyl and amino groups of adjacent amino acids to from long, unbranched molecules. Such bonds are commonly called peptide bonds. The structures resulting from the formation of peptide bonds are called dipeptides, tripeptides, or polypeptides, depending on the number of amino acids involved. The individual amino acids are called residues. Biuret, a simple molecule prepared from urea, contains what may be regarded as two peptide bonds and thus is structurally similar to simple peptides. This molecule when treated with copper sulfate in alkaline solution (the biuret reaction) gives an intense purple color. The reaction is based on the formation of a purple-colored complex between copper ions and two or more peptide bonds. Proteins give a particularly strong biuret reaction because they contain a large number of peptide bonds. The biuret reaction may be used to quantitate the concentration of proteins because peptide bonds occur with approximately the same frequency per gram of material for most proteins. In this experiment, you will determine the concentration of unknown protein solutions by measuring colorimetrically the intensity of their color production in the biuret reaction as compared with the color produced by a known concentration of the protein albumin. Page 36 of 40 Name: ______________________________ AP Biology – Lab 02 Calibration of the Spectrophotometer Figure 11: Diagram of a spectrophotometer. 1. Rotate the wavelength control (1) shown in Figure 11 until the desired wavelength is shown on the wavelength dial (2). The wavelength for a given substance can be found by referring to the literature or by determining it experimentally. 2. Turn the instrument on by rotating the ―0‖ control (3) in a clockwise position. Allow at least 5 minutes for the instrument to warm up. 3. Adjust the ―0‖ control with the cover of the sample holder closed (5) until the needle is at 0 on the tranmittance scale (4). 4. Place a cuvette containing water or another solvent in the sample holder, and close the cover. 5. Rotate the light control (6) so that the needle is at 100 on the transmittance scale (0.0 absorbance). This control regulates the amount of light passing through the second slit through the phototube. 6. The unknown samples may then be placed in the tube holder, and the percentage of transmittance or absorbance can be read. The needle should always return to zero when the tube is removed. Check the 0% and 100% transmittance occasionally with the solvent tube in the sample holder to make certain the unit is calibrated. Note: Always check the wavelength scale to be certain that the desired wavelength is being used. Page 37 of 40 Name: ______________________________ AP Biology – Lab 02 Using a Standard Curve to Determine an Unknown 7. Prepare a set of six test tubes, containing increasing amounts of a standard solution of albumin and 0.5 M KCl, as shown in Table 19. Table 19: Preparing a standard curve for albumin. Tube Number Albumin 2.5 mg/mL (mL) Total protein content (mg) 0.5 M KCl (mL) Biuret reagent (mL) %T A540 1 0.0 0.0 5.0 0.5 100 0.0 2 1.0 2.5 4.0 0.5 3 2.0 5.0 3.0 0.5 4 3.0 7.5 2.0 0.5 5 4.0 10.0 1.0 0.5 6 5.0 12.5 0.0 0.5 ( ) --- 0.5 ( ) --- 0.5 8. Add 0.5 mL of biuret reagent to each of the tubes, and mix thoroughly by rotating the tubes between your palms. The color is fully developed in 20 minutes and is stable for at least an hour. While waiting for the color to develop, calibrate your instrument at 540 nm using Tube 1—which is a blank containing all reagents except protein. 9. Read Tubes 2 through 6 against it. Record your readings in Table 19. Covert the % T to absorbance (A) for Tubes 1 through 6 using Table 20 and then plot your data to obtain a standard curve for the total protein content of albumin (not volume of albumin). Do this on the graph paper provided, and also use Excel to do the same (for Excel, do a regression analysis to check the reliability of your standard curve). 10. Using the standard curve, determine the concentrations of your unknown protein solutions using both your handwritten graph and the Excel spreadsheet/graph. Put these values on the above table. Page 38 of 40 Name: ______________________________ AP Biology – Lab 02 Table 20: Absorbance vs. Transmittance. %T 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 Absorbance (A) 2.000 1.699 1.532 1.398 1.301 1.222 1.155 1.097 1.046 1.000 .959 .921 .886 .854 .824 .796 .770 .745 .721 .699 .678 .658 .638 .620 .602 .585 .569 .553 .538 .532 .509 .495 .482 .469 .456 .444 .432 .420 .409 .398 .387 .377 .367 .357 .347 .337 .328 .319 .310 .301 %T .25 .50 .75 1.903 1.648 1.488 1.372 1.280 1.204 1.140 1.083 1.034 .989 .949 .912 .878 .846 .817 .789 .763 .739 .716 .694 .673 .653 .634 .615 .598 .581 .565 .549 .534 .520 .505 .491 .478 .465 .453 .441 .429 .417 .406 .395 .385 .374 .364 .354 .344 .335 .325 .317 .308 .299 1.824 1.602 1.456 1.347 1.260 1.187 1.126 1.071 1.022 .979 .939 .903 .870 .838 .810 .782 .757 .733 .710 .688 .668 .648 .629 .611 .594 .577 .561 .545 .530 .516 .502 .488 .475 .462 .450 .438 .426 .414 .403 .392 .385 .372 .362 .352 .342 .332 .323 .314 .305 .297 1.757 1.561 1.426 1.323 1.240 1.171 1.112 1.059 1.011 .969 .930 .894 .862 .831 .803 .776 .751 .727 .704 .683 .663 .643 .624 .606 .589 .573 .557 .542 .527 .512 .496 .485 .472 .459 .447 .435 .423 .412 .401 .390 .380 .369 .359 .349 .340 .330 .321 .312 .303 .295 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 Absorbance (A) .2924 .2840 .2756 .2676 .2596 .2518 .2441 .2366 .2291 .2218 .2147 .2076 .2007 .1939 .1871 .1805 .1739 .1675 .1612 .1549 .1487 .1427 .1367 .1308 .1249 .1192 .1135 .1079 .1024 .0969 .0975 .0862 .0809 .0757 .0706 .0655 .0605 .0555 .0505 .0458 .0410 .0362 .0315 .0269 .0223 .0177 .0132 .0088 .0044 .0000 .25 .50 .75 .2903 .2819 .2736 .2656 .2577 .2499 .2422 .2347 .2273 .2200 .2129 .2059 .1990 .1922 .1855 .1788 .1723 .1659 .1596 .1534 .1472 .1412 .1652 .1293 .1235 .1177 .1121 .1065 .1010 .0955 .0901 .0848 .0796 .0744 .0693 .0672 .0593 .0543 .0494 .0446 .0396 .0351 .0304 .0257 .0212 .0166 .0121 .0077 .0033 .0000 .2882 .2798 .2716 .2636 .2557 .2480 .2403 .2328 .2255 .2182 .2111 .2041 .1973 .1905 .1838 .1772 .1707 .1643 .1580 .1517 .1457 .1397 .1337 .1278 .1221 .1163 .1107 .1051 .0996 .0942 .0888 .0835 .0783 .0731 .0680 .0630 .0580 .0531 .0482 .0434 .0386 .0339 .0292 .0246 .0200 .0155 .0110 .0066 .0022 .0000 .2861 .2777 .2696 .2616 .2537 .2460 .2384 .2319 .2236 .2164 .2093 .2024 .1956 .1888 .1821 .1756 .1691 .1627 .1565 .1503 .1442 .1382 .1322 .1264 .1206 .1149 .1093 .1037 .0982 .0928 .0875 .0822 .0770 .0718 .0667 .0617 .0568 .0518 .0470 .0422 .0374 .0327 .0281 .0235 .0188 .0144 .0099 .0055 .0011 .0000 Note: Intermediate values may be arrived at by using the .25, .50, and .75 columns. For example, if %T equals 85, the absorbance equals .0706. If %T equals 85.75 the absorbance equals .0067 Page 39 of 40 Name: ______________________________ Page 40 of 40 AP Biology – Lab 02