Pharmacokinetics 5 Metabolism
advertisement

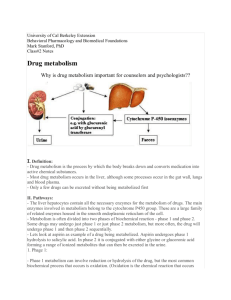
Metabolism Lecture 6 Dr Maura Grealy Most drugs: • • • • • • • Leave the body in the urine: UNCHANGED or as Polar metabolites Some secreted into bile via liver (most reabsorbed via GIT) Some faecal elimination (e.g. rifampicin) Some excretion via lungs (volatile/gaseous agents) Some excretion occurs in sweat or milk (nursing mothers) Metabolism (Biotransformation) Can alter a drug in 4 important ways Convert an active drug into an inactive drug Convert an active drug to an active or toxic metabolite Convert an inactive prodrug to an active drug Convert an unexcretable drug to an excretable metabolite Inactive prodrugs have been developed to A. B. C. D. reduce drug toxicity increase drug half-life decrease hepatic drug metabolism increase drug absorption Lipophilicity To be excreted by kidneys in urine a drug needs to be Lipophilic drugs Hydrophilic? Hydrophobic (lipophilic)? Not eliminated efficiently by kidney Biotransformation into less lipophilic compounds Polar metabolites Aids elimination in the kidney Drug Elimination Irreversible loss of drug from the body Main Routes Kidneys Hepatobiliary system lungs 2 processes Metabolism Excretion Metabolism Primary goal is drug inactivation Primary site is the liver Uses enzymes to convert drug from one form to another (usually less active) form Enzymes = cytochrome P450 system Large group of related enzymes in the liver Main job is detoxifying foreign chemicals including drugs Divided into Phase I reactions Phase II reactions Chemical reactions in drug metabolism Oxidation Reduction Hydrolysis Conjugation Result in the formation of polar metabolites both less active than, and more readily excreted than, the parent compound 2 phases of drug metabolism Phase 1 – usually precedes phase 2 (“Functionalisation”) Oxidation Reduction Hydrolysis CATABOLIC Products often more chemically reactive (paradoxically sometimes more toxic or carcinogenic than the parent drug) Hydrolysis Phase 2 of drug metabolism Biosynthetic ANABOLIC Conjugation (allows attachment of a group) Usually results in inactive products Site of drug metabolism Liver Epithelial cells of small intestine High concentrations of many drug metabolizing enzymes Expression of certain isoforms Kidney, Lung, Skin also Liver enzymes Embedded in smooth endoplasmic reticulum Microsomal enzymes (because ER breaks into small fragments called microsomes on homogenisation and centrifugation) Drug must cross plasma membrane to get there Thus more NB for lipophilic drugs Phase I Reactions Cytochrome P450 enzymes Differ from each other Haem proteins Large superfamily of related enzymes CYP + number Amino acid sequence Sensitivity to inhibitors / inducers Specificity of reactions they catalyse 3 main genes are CYP1, CYP2, CYP3 CYPs CYtochrome P450 Family) CYP1 Family: CYP2 Family: Primarily metabolize carcinogens, some drugs as well. Metabolize many important drugs CYP3 Family: Most abundant family in human liver, metabolize many important drugs Phase I reactions - Oxidation Drug + O2 + NADPH + H+ Drug-OH + H2O + NADP+ PHASE 1 Oxidation reactions Hydroxylation -CH2CH3 -CH2CH2OH Eg barbiturates, cyclosporine Oxidation -CH2OH -CHO Eg methanol, ethanol N-de-alkylation -NHCH3 -COOH -NH2 + CH2O e.g methamphetamine, lidocaine Oxidative deamination -CHCH3 -COCH3 + NH3 | NH2 e.g histamine, noradrenaline Phase I in action Imipramine N CH2CH2CH2NCH3CH3 Phase I in action Imipramine N desmethyl imipramine (antidepressant) CH2CH2CH2NCH3CH3 2-hydroxy imipramine (cardiotoxic) Phase I rxns not involving CYP450 95% of oxidation biotransformations are via CYP 450 Others Alcohol dehydrogenase Methanol to formaldehyde (toxic) Monamine oxidase Oxidation of catecholamines and tyramine Reduction (electron gain) Addition of H to N, O, or C= Nitro groups changed to an amine Carbonyl groups changed to a hydroxyl RNO2 RNH2 eg chloramphenicol RC=O RC-OH eg haloperidol; methadone Enzymes for Reduction Aldo-keto reductase gene superfamily CYP family can also participate in reduction reactions Eg halothane (anaesthetic) De-halogenated R-X R-H Warfarin by CYP2A6 Hydrolysis Drugs containing esters RCOOR’ RCOOH +HOR’ Eg procaine, succinylcholine, aspirin Hydrolysed by non-specific esterases In liver, plasma, GIT Hydrolysis Amides RCONHR’ Procainamide, lidocaine Hydrolysed by amidases RCO-OH +H2NR’ In liver Polypeptide drugs eg insulin, growth hormone Transformed by peptidases In plamsa, red blood cells, + many tissues Hepatic cytochrome P450 drugmetabolizing enzymes are primarily found in A. B. C. D. cell nuclei plasma membranes the cytoplasm the smooth endoplasmic reticulum