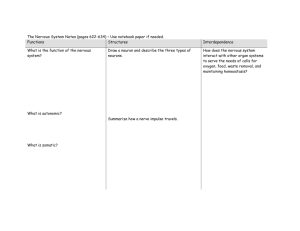
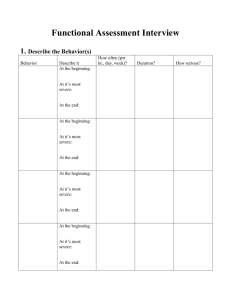
Phylogenetic origins of affective experiences
advertisement

In D. Fosha, D. J. Siegel, & M. F. Solomon (Eds.) (2009, in press). The healing power of emotion: Affective neuroscience, development, clinical practice. New York: Norton Please do not cite without author permission Reciprocal influences between body and brain in the perception and expression of affect: A polyvagal perspective Stephen W. Porges, Ph.D. Brain-Body Center (MC 912) Department of Psychiatry University of Illinois at Chicago 1601 W. Taylor Street Chicago, IL 60612 sporges@uic.edu Introduction Emotions, affect regulation, and interpersonal social behavior are psychological processes that describe basic human experiences in response to events, environmental challenges and people. These processes shape our sense of self, contribute to our abilities to form relationships, and determine whether we feel safe in various contexts or with specific people. Although these processes can be objectively observed and subjectively described, they represent a complex interplay between our psychological experience and our physiological regulation. These psychological-physiological interactions are dependent on the dynamic bidirectional communication between peripheral organs and the central nervous system connecting the brain with these organs. For example, the neural circuits, providing a bidirectional communication between the brain and heart, can trigger either a rapid increase in heart rate to support protective fight/flight behaviors or a rapid decrease in heart rate to support social interactions. Peripheral physiological reactions can be initiated by the brain detecting features of danger in the environment and alternatively, changes in peripheral physiological state can feedback to the brain and alter our perceptions of the world. Thus, affect and interpersonal social behavior are more accurately described as biobehavioral than psychological processes, since our physiological state can profoundly influence the quality of these psychological processes and our feelings can, in turn, determine dynamic changes in our physiology. Our nervous system functions as a sentry by continuously evaluating risk in the environment. Through neural surveillance mechanisms (i.e., neuroception), our brain identifies features of risk or safety. Many of the features of risk and safety are not learned, but are hardwired into our nervous system and reflect adaptive strategies associated with our phylogenetic history. For example, low frequency sounds elicit in mammals a sense of danger associated with approaching predator. This reaction is shared with other vertebrates including reptiles and amphibians. Due to our phylogenetic history, the rumble of low frequency sounds shifts our attention from social interactions to potential dangers in the environment. In contrast, high-pitched screams from another mammal (not just our children, but also our dogs and cats) elicit a sense of urgent concern or empathy for another who may be feeling pain or being injured. With humans high frequency screams shift our attention to the specific individual who is screaming. Through exposure and associative learning, we can link these features with other events. Specific features in the environment recruit physiological states differentially associated with feelings of safety, danger, or ultimate demise (i.e., life threat). Each of these states is characterized by a specific set of capacities for affect regulation, and social engagement and communication (Porges, 2003). Current research in affective neuroscience focuses on brain structures and neural circuits related to specific motivational and emotional processes (e.g., Panksepp, 1998). These important discoveries emphasize cortical and subcortical structures in the emergence of the complex affective S.W. Porges The Healing Power of Emotion (2009,in press) page 2 repertoire of humans and their contribution to social relationships (e.g., Schore, 1994, 2003; Siegel, 2007). However, underlying these contributions are details of an important and often overlooked neurobiological substrate, the neural circuits mediating the reciprocal communication between body states and brainstem structures, which impact on the availability of these affective circuits. These underlying circuits not only promote feelings (e.g., Damasio, 1999), but also form a bidirectional circuit (e.g., Darwin, 1872) that enables mental and psychological processes to influence body state and body state to color and, at times, to distort our perception of the world. Thus, the study of affective processes, especially in their prosocial and healing roles, requires an understanding of the neural circuits both between higher brain structures and the brainstem and between the brainstem and the visceral organs (e.g., the heart) mediated through the autonomic nervous system. All affective or emotional states are dependent upon lower brain regulation of the visceral state and the important visceral, tactile, and nocioceptive cues that travel to the brain from the periphery. Moreover, there are distinct visceral regulatory states that foster different domains of behavior. These states do not preclude the important bidirectional information from higher brain structures. This chapter will emphasize the neural regulation of observable facial movements and concurrent subjective visceral experiences that characterize the expressions, feelings, and perceptions of emotion and affective state. The chapter will use the Polyvagal Theory (Porges, 1995, 1997, 1998, 2001a, 2003, 2007) as an organizing principle to explain the role of visceral state in the accessibility of prosocial emotions and restorative affective states. The Polyvagal Theory is an attempt to reorganize our conceptualization of the autonomic nervous system with a focus on the specific neural circuits involved in regulating visceral organs for specific adaptive functions related to affect, emotions, and social communication behaviors. The Polyvagal Theory interprets social interactions and emotion as biobehavioral processes. Thus, the theory is particularly important for psychotherapists, who focus on the social interaction within the therapeutic setting and forego pharmacological interventions. By treating the social interaction as a biobehavioral process, it is possible to conceptualize a therapeutic treatment that relies, not on pharamacological manipulations, but on the profound positive impact of social interactions and interpersonal behaviors on the neural regulation of body state and behavior. By exploring these bidirectional biobehavioral processes, psychotherapeutic treatments may change the neural regulation of physiological state, which in turn will support further benefits from interpersonal interactions. Emotion, motion, and visceral state: Features of mental health Regardless of the operational, and often arbitrary, distinction between emotion and affect or between emotional expressions and feelings, the measurement of physiological state (e.g., autonomic, endocrine, and muscle activity) needs to be embraced in affective neuroscience, particularly if there is to be a functional dialogue with experiential clinicians. In most cases physiological state has been conceptualized as a correlate or a consequence of higher brain structures (e.g., cortex) presumed to be driving emotion and affect. However, it would be naïve not to explore the connections and potential bidirectional influences between peripheral physiological state and the brain circuits related to affective processes. Physiological state is an implicit component of the subjective experiences associated with specific psychological constructs such as anxiety, fear, panic, and pain. The convergence between physiological state and emotional experience is neurophysiologically determined, since the metabolic requirements necessary to modulate the muscles of the face and body require supporting 2 S.W. Porges The Healing Power of Emotion (2009,in press) page 3 changes in autonomic state. All emotional and affective states require specific physiological shifts to facilitate their expression and to reach their implicit goals (e.g., fight, flight, freeze, proximity, etc). Through the study of phylogenetic shifts in the vertebrate autonomic nervous system, it is possible to link the different expressive features of emotion in humans with the phylogenetic transitions in visceral regulation observed in vertebrates. Physiological monitoring provides an important portal to monitor these reactions, since some affective responses are often not observable in overt behavior. For example, the convergence between the neural mechanism mediating autonomic state and facial expressions phylogenetically occurs in the transition from reptiles to mammals (see Porges, 1995, 2007). There is a rich history of research linking the neural regulation of face and viscera (e.g., heart) with brain circuits. Gellhorn (1964) elaborated on how proprioceptive discharges from facial muscles influence brain function and promote changes in visceral state. Thus, providing an example of the bidirectionality between peripheral and central structures and providing a neurophysiological basis for the assumed relation between facial expression and body feelings. Even earlier, Darwin (1872) acknowledged the important and often neglected bidirectional relation between the brain and the heart in this quote from The Expression of Emotions in Man and Animals, “…when the heart is affected it reacts on the brain; and the state of the brain again reacts through the pneumo-gastric [vagus] nerve on the heart; so that under any excitement there will be much mutual action and reaction between these, the two most important organs of the body (p. 69).” Although Hess (1949) was awarded the Nobel i prize in Physiology or Medicine for his work emphasizing the importance of the central regulation of visceral state, journals in contemporary affective neuroscience (e.g., Nature Neuroscience) and biological psychiatry (e.g., Biological Psychiatry or CNS) express a disconnect between subjective affective experience and visceral state regulation. Contemporary affective neuroscience with the aids of both imaging techniques and neurochemistry has focused on brain structures contributing to various neural circuits involved in adaptive behaviors with apparent motivational objectives. Panksepp (1998; also this volume) organizes affective experiences into seven neural-based motivational systems that include Seeking, Rage, Fear, Lust, Care, Panic, and Play. However, missing from these functionally adaptive motivational circuits is the role that neural regulation of visceral state plays in potentiating or dampening these circuits. For example, if an individual is in a physiological state characterized by vagal withdrawal and high sympathetic excitation, body feelings of a fast pounding heartbeat are experienced and the threshold to react aggressively is low. In contrast, when in a physiological state characterized by an engaged myelinated vagus, sympathetic and HPA-axis reactivity are dampened. The physiological state is experienced as “calm.” Intrusive stimuli that previously would have triggered aggressive behaviors when the vagal activity is withdrawn will now result in a dampened reaction. Accompany this change in physiological state are options to further dampen reactivity through social interactions. Most proponents of affective neuroscience embrace a science of parallelism that links either observable emotional expressions or subjective experiences with a “neural” specificity that is concretized and assumed to be validated by imaging studies that identify activation of brain areas or blockade studies interfering with appropriate functioning of these circuits. Thus, to many affective neuroscientists, affect resides solely in the brain and does not require inputs or outputs linking the 3 S.W. Porges The Healing Power of Emotion (2009,in press) page 4 body to the brain. Missing from this research agenda and theoretical explanation is an appreciation of the necessary contributions of both the sensory inputs and the motor outputs from the central circuits. Focusing on the central circuits, without studying the sensory and motor contributions, is like studying the behavior of a thermostat independent of information regarding both ambient temperature and the capacity of the heating, ventilation, and air conditioning system. Hess was aware that, although the components of a feedback circuit might be identified and studied independently, the functioning of independent parts did not explain how the system, as a whole, functioned dynamically during the moment-to-moment challenges of life. This limitation was, in part, dependent on the methodologies of the day that required pharmacological, surgical, or electrical manipulations to block or stimulate “global” branches of the autonomic nervous system that either shared a specific neurotransmitter (e.g., acetylcholine, epinephrine) or an easily identifiable nerve (e.g., vagus) that could be cut or stimulated. Within the field of mental health, there is a similar acceptance of a disease model without a focus on the intervening feedback circuits that mediate the features of the disorder. Within psychiatry, anxiety and depression are defined by clinical features and not by a measurable physiological substrate. The prevalent strategies in mental health research that use neurophysiological variables (e.g., imaging, autonomic measures) are not directed at defining anxiety or depression, but use neurophysiological variables as correlates of a clinical diagnosis. The value of taking a different perspective can be illustrated with the construct of anxiety. If anxiety were viewed as dependent on a shift in autonomic state in which an individual’s physiological state is dominated by the sympathetic nervous system, new clinical research strategies might emerge that focus on characterizing how states of anxiety and a vulnerability to being anxious would be potentiated or dampened by different autonomic states. Treatments would then be developed either to (i) dampen sympathetic tone or (ii) enable the individual to move to environments or shift contexts that are less likely to trigger the increased reactivity associated with higher sympathetic excitation. Unfortunately, most researchers in psychiatry and psychology express little interest in the mapping autonomic regulation as a "vulnerability" dimension for various psychiatric disorders and behavioral problems, although visceral features are often symptoms of the disorders they are treating. Clinical disciplines rarely acknowledge the proximal functions of visceral state. Clinicians seldom monitor the expression of vagal withdrawal or sympathetic excitation in their patients. Such a shift in autonomic state would be manifested in several physical and psychiatric symptoms including flat affect, difficulties in auditory processing, hyperacusis, tachycardia, and constipation. In addition, conventional models of mental disorders neglect the role of neurophysiological mechanisms dynamically interacting with contextual cues in the environment. In contrast, these disciplines have embraced distal constructs related to the functions of receptors within the brain that lead almost reflexively to drug treatment, while generally failing to recognize the important role of visceral state and visceral afferent feedback on the global functioning of the brain. This strategy is far from parsimonious and does not take into account either the phylogeny of the mammalian nervous system or the intervening neurophysiological and biobehavioral systems along a continuum from genes to behavior. Rather these disciplines have assumed that clusters of observable behaviors or subjective experiences are linked parsimoniously and directly to neurochemical levels in specific brain circuits. Thus, missing the important potential of psychological and behavioral interventions (including changes in environment) that would be therapeutic by directly influencing physiological state without necessitating pharmacological treatments. 4 S.W. Porges The Healing Power of Emotion (2009,in press) page 5 State regulation and the autonomic nervous system: A historical perspective Researchers for over a century have measured autonomic variables (e.g., heart rate, palmar sweat gland activity) as indicators of emotional state related to perceived stress (e.g., fear, mental effort, workload, and anxiety). Historically, arousal theories (e.g., Berlyne, 1960; Gray, 1971; Darrow, 1943) provided scientists who study brain-behavior relations with a model that assumed that activation of peripheral physiological measures regulated by the sympathetic branch of the autonomic nervous system were sensitive indicators of brain "arousal" or "activation." This view was based on a rudimentary understanding of the autonomic nervous system in which changes in easily measured peripheral organs (e.g., sweat glands, heart) were assumed to be accurate indicators of how the brain was processing emotional stimuli. Usually, emotional states were associated with fight-flight behaviors and the sympathetic-adrenal system (e.g., increases in heart rate, sweat gland activity, and circulating catecholamines) as initially described by Cannon (1928). Based on Selye (1936, 1956), emotional states were also associated with increased activity of the hypothalamicpituitary-adrenal (HPA) axis (e.g., increases in cortisol). From a psychological level, arousal theories emphasized fight-flight behaviors and neglected or minimized the importance of both prosocial affective states that facilitated social interaction and also the defensive strategy of immobilization (e.g., fainting, death feigning). An acceptance of a unitary arousal system in assumed in several research domains, including investigations of sleep, deception, sexual behavior, and anxiety. Moreover, it led to research on cortical “arousal” and the use of EEG, SPECT, fMRI, and other imaging technologies that accepted the arousal construct with little interest in the distinction between activation of neural pathways that were excitatory or inhibitory. This resulted in difficulties in establishing whether “activation” represents the turning on or the turning off of a specific neural structure. From a physiological level, arousal theories emphasize an assumed continuity between central cortical activation and peripheral arousal marked by increases in the activity of the sympathetic nervous system and the adrenal hormones. However, arousal theories have neglected both the importance of the parasympathetic branch of the autonomic nervous system and the bidirectional communication between brain structures and visceral organs. The continuity between brain and peripheral arousal created a research environment that neglected several important factors including: an understanding of the brain structures that regulate autonomic function; how these structures evolved from the most primitive vertebrates to mammals; how the autonomic nervous system interacts with the immune system, the HPA axis, and the neuropeptides, oxytocin and vasopressin; and the co-evolution of stress and coping strategies with the increasing complexity of the autonomic nervous system. Missing from this dialog is a discussion of the role of the parasympathetic nervous system and especially the vagus (the 10th cranial nerve) with its bidirectional portal between the brain and specific visceral organs such as the heart. The Polyvagal Theory: A primer The Polyvagal Theory emerged from the study of the evolution of the vertebrate autonomic nervous system. The theory assumes that many of our social behaviors and vulnerabilities to emotional disorders are “hardwired” into our nervous system. Based on the theory, it is possible to understand various aspects mental health and to develop treatment techniques that can help people communicate better and relate better to others. The term “polyvagal” combines “poly,” meaning “many,” and “vagal,” which refers to the important nerve called the “vagus.” To understand the 5 S.W. Porges The Healing Power of Emotion (2009,in press) page 6 theory, we need to investigate features of the vagus nerve, a primary component of the autonomic nervous system. The vagus nerve exits the brain stem and has branches that regulate several organs, including the heart. The theory proposes that there are two branches of the vagus are related to different behavioral strategies, one related to social interactions in safe environments and the other related to adaptive responses to life threat. Historically, the autonomic system has been broken into two opposing components, one labeled sympathetic, and the other parasympathetic. This organizational model was used to describe the function of the autonomic nervous system in the late 1800s and the early 1900s. In the 1920s this paired-antagonism model was formalized (Langley, 1921). This model characterized the function of the autonomic nervous system as a constant battle between the sympathetic nervous system associated with fight/flight behaviors and the parasympathetic nervous system associated with growth, health, and restoration. Because most organs of the body, such as the heart, the lungs and the gut, have innervations from both sympathetic and parasympathetic components, the pairedantagonism model evolved into “balance theories.” Balance theories attempted to link “tonic” imbalances to both physical and mental health. For example, a sympathetic dominance might be related to symptoms of anxiety, hyperactivity, or impulsivity, while a parasympathetic dominance might be related to symptoms of depression or lethargy. In addition to the tonic features of autonomic state, the pair-antagonism model also was assumed to explain the reactive features of the autonomic nervous system. This dependence on the construct of “autonomic balance” is still prevalent in textbooks, although there has been an intervening century in which neurophysiology has documented a second vagal pathway involved in regulating autonomic function. Unfortunately, this new knowledge of the second vagal pathway has not permeated the teaching of physiology, which still is dominated by descriptions of the paired-antagonism between the sympathetic and parasympathic components of the autonomic nervous system. The primary parasympathetic influence to peripheral organs is conveyed through the vagus, a cranial nerve that exits the brain and innervates the gastrointestinal tract, respiratory tract, heart and abdominal viscera. The vagus can be conceptualized as a tube or conduit containing several sensory and motor fibers originating or terminating in different areas of the brainstem. For example, vagal motor pathways that regulate the lower gut originate in the dorsal nucleus of the vagus (DMX), the vagal pathways that regulate the heart and the lungs originate in the nucleus ambiguus, and the vagal pathwasy sending sensory information from the gut terminate in the nucleus of the solitary tract (NTS) . The Polyvagal Theory proposes that the autonomic nervous system reacts to real world challenges in a predictable hierarchical manner that parallels, in reverse, the phylogenetic history of the autonomic nervous system in vertebrates. In other words, if we study the evolutionary path of how the autonomic nervous system unfolded in vertebrates (i.e., from ancient jawless fish to bony fish, amphibians, reptiles, and mammals), we learn not only that there is an increase in the growth and complexity of the cortex, (the outer layer of the cerebrum), but also that there is a change in composition and function of the autonomic nervous system. In mammals, the autonomic nervous system functions as a hierarchical system that parallels phylogenetic states in reverse and not as the balance between sympathetic/ parasympathetic systems. The phylogenetic changes in the autonomic nervous system (including changes in neural pathways and brainstem areas regulating the peripheral organs) determine how the autonomic nervous system reacts to challenges. In humans and other mammals, the hierarchy is composed of 6 S.W. Porges The Healing Power of Emotion (2009,in press) page 7 three neural circuits with the newer circuits having the capacity to override the older circuits. Under most challenges in our environment, we initially react with our newest system (i.e., myelinated vagus). If that circuit does not satisfy our biobehavioral quest for safety, an older circuit spontaneously reacts (i.e., sympathetic nervous system). Finally, if the former strategies are unsuccessful, as our last option we reflexively trigger the oldest circuit (i.e., unmyelinated vagus). Functionally, in humans the older vagal circuit is involved in adaptive reactions characterized by immobilization and decrease in metabolic resources, while the newer vagal circuit is involved in regulating calm states that promote both spontaneous social engagement and health, growth, and restoration. communication. Along the phylogenetic hierarchy, between the two vagal circuits, is the sympathetic nervous that supports fight and flight behaviors. The Polyvagal Theory: The biobehavioral quest for safety, survival, and a painless death To survive mammals must determine friend from foe, when an environment is safe, and communicate to their social unit. These survival-related behaviors limit the extent to which a mammal can be physically approached, whether vocalizations will be understood, and whether coalitions can be established. Moreover, these behavioral strategies, which are used to navigate through the “stress of life,” form the bedrock upon which social behaviors and higher cognitive processes can be developed and expressed. Thus, learning and other expansive mental processes must be structured, manipulated and studied within the context of how the environment fosters or ameliorates stress-related physiological states. The Polyvagal Theory proposes that the evolution of the mammalian autonomic nervous system provides the neurophysiological substrates for affective processes and stress responses. The theory proposes that physiological state limits the range of adaptive behaviors and psychological experiences. Thus, the evolution of the nervous system determines the range of emotional expression, quality of communication, and the ability to regulate body and behavioral state including the expression and recovery of stress-related responses. Relevant to adaptive social and emotional behaviors, these phylogenetic principles illustrate the emergence of a brain-face-heart circuit and provide a basis for investigating the relation between several features of mental health and autonomic regulation. Via evolutionary processes, the mammalian nervous system has emerged with specific features that react to challenge to maintain visceral homeostasis. In general, the domains of homeostasis, which have been monitored, have focused on the visceral systems involved in cardiovascular, digestive, reproductive and immune functions. For example, studies have evaluated how long it takes heart rate to recover following a challenge to a pre-stress level. Adaptive coping requires minimizing the magnitude and duration of this deviation, whether the deviation is observed in raising heart rate, blood pressure, cortisol, or disrupting digestion. By investigating the phylogeny of the regulation of the vertebrate heart (e.g., Morris and Nilsson, 1994), three principles can be extracted. First, there is a phylogenetic shift in the regulation of the heart from endocrine communication, to unmyelinated nerves, and finally to myelinated nerves. Second, there is a development of opposing neural mechanisms of excitation and inhibition to provide rapid regulation of graded metabolic output. Third, with increased cortical development, the cortex exhibits greater control over the brainstem via direct (e.g., corticobulbar) and indirect (e.g., corticoreticular) neural pathways originating in motor cortex and terminating in the source nuclei of the myelinated motor nerves emerging from the brainstem (e.g., specific neural pathways embedded within cranial nerves V, VII, IX, X, XI), controlling visceromotor structures 7 S.W. Porges The Healing Power of Emotion (2009,in press) page 8 (i.e., heart, bronchi, thymus) and somatomotor structures (muscles of the face and head) that results in a neural circuit that functions to facilitate social behavior and to maintain calm behavioral states. These phylogenetic principles illustrate the emergence of a brain-face-heart circuit and provide a basis for investigating the relation between several features of mental health and autonomic regulation. In general, phylogenetic development results in increased neural control of the heart via the myelinated mammalian vagal system that is paralleled by an increased in the neural regulation of the facial muscles. This integrated system can “cue” others of safety and danger, while promoting transitory mobilization and the expression of sympathetic tone without requiring sympathetic or adrenal activation (i.e., raising heart rate by the removing the myelinated vagal inhibition from the heart). Functionally, this phylogenetic progression provides a system that can respond rapidly (i.e., via myelinated pathways), selectively regulate the magnitude (i.e., via opposing inhibitory and excitatory circuits), and specificity of the features (e.g., via calming or excitation and linkage of autonomic reactivity with facial muscles) of the reaction. With this new vagal system, transitory incursions into the environment or withdrawals from a potential predator can be initiated without the severe biological cost of the metabolic excitation associated with sympathetic-adrenal activation. Paralleling this change in neural control of the heart is an enhanced neural control of the face, larynx, and pharynx that enables complex facial gestures and vocalizations associated with social communication. This phylogenetic course results in greater central nervous system regulation of behavior, especially behaviors needed to engage and disengage rapidly with environmental challenges. These phylogenetic shifts, which promote a greater bidirectional communication between brain and viscera, provide opportunities for mental processes, including voluntary behavior, to impact on body state. Thus, a greater understanding of the circuit mediating these interactions might lead to functional models of intervention that would both calm visceral state and promote more prosocial interactions. Consistent with this trend, new research and clinical programs are emerging. For example, Cleveland Clinic has created the Bakken Heart-Brain Institute and has run annual Heart-Brain Institute Summit to bring “together researchers, clinicians and others to stimulate greater collaboration and understanding of the heart-brain link and to positively impact research, education, and patient care.” Three phylogenetically defined autonomic circuits supporting adaptive behaviors The Polyvagal Theory emphasizes and documents the neurophysiological and neuroanatomical distinction between the two branches of the vagus (i.e., tenth cranial nerve) and proposes that each vagal branch is associated with a different adaptive behavioral and physiological response strategy to stressful events. The theory describes three phylogenetic stages of the development of the mammalian autonomic nervous system. These stages reflect the emergence of three distinct subsystems, which are phylogenetically ordered and behaviorally linked to social engagement, mobilization, and immobilization. The phylogenetic orientation focuses our interest on the parasympathetic neural structures and neurobehavioral systems that we share with or have adapted from our phylogenetic ancestry. With increased neural complexity, due to phylogenetic development, the organism’s behavioral and affective repertoire is enriched. The Polyvagal Theory (Porges, 1995, 1997, 1998, 2001a, 2003, 2007) emphasizes the phylogenetic origins of brain structures that regulate social and defensive behaviors. For example, prosocial behaviors cue others that the environment is safe. Safe environments signal the individual to dispense with the hypervigilance required to detect danger and allows this precautionary strategy to be replaced with social interactions that further calm and lead to close proximity and physical contact. The prototypical prosocial behaviors in mammals are related to nursing, reproduction, 8 S.W. Porges The Healing Power of Emotion (2009,in press) page 9 interactive play, and being able to be calm in the presence of another. In contrast, defensive behaviors could be categorized into two domains: one related to mobilization including fight and flight behaviors and the other related to immobilization and death feigning that might be associated with dissociative psychological states. Within this dichotomy of defensive strategies, freezing behavior that requires increased muscle tension in the absence of movement, such as stalking or vigilance behaviors, is categorized within mobilization. In contrast, immobilization is associated with a decrease in muscle tension and often with fainting and other features of decreased metabolic activity. From a health perspective, the prosocial behaviors trigger neurophysiological circuits that not only support affect regulation and social interactions, but also promote health, growth, and restoration. Relevant to adaptive social and emotional behaviors, the Polyvagal Theory makes the following assumptions: 1. Evolution has modified the structures of the autonomic nervous system. 2. The mammalian autonomic nervous system retains vestiges of phylogenetically older autonomic nervous systems. 3. Emotional regulation and social behavior are functional derivatives of structural changes in the autonomic nervous system due to evolutionary processes. 4. In mammals, the autonomic nervous system response strategy to challenge follows a phylogenetic hierarchy, starting with the newest structures and, when all else fails, reverting to the most primitive structural system. 5. The phylogenetic stage of the autonomic nervous system determines the behavioral, physiological, and affective features of reactivity to people and objects in the environment. The phylogenetic orientation focuses our interest on the parasympathetic neural structures and neurobehavioral systems that we share with or have adapted from our phylogenetic ancestry. First, there are three response systems proposed in the Polyvagal Theory: 1) cranial nerves to regulate the face and to mediate calm autonomic and behavioral states, 2) sympathetic-adrenal system to increase metabolic output, and 3) an inhibitory vagal system to decrease metabolic output and promote freezing and defecation. These three response strategies are the products of distinct neurophysiological systems. Second, these distinct neurophysiological systems represent a phylogenetically-dependent hierarchy with the use of cranial nerves to regulate facial expression emerging in mammals (well developed in primates), the sympathetic-adrenal system shared with other vertebrates including reptiles, and the inhibitory vagal system shared with more primitive vertebrates including amphibians, bony fish, and cartilaginous fish (see Porges, 1997, 1998). The three systems represent different phylogenetic stages of neural development. This phylogenetic development starts with a primitive behavioral inhibition system, progresses to a fight-flight system, and, in humans (and other primates), culminates in a complex facial gesture and vocalization system. Thus, from a phylogenetic perspective, the nervous system of vertebrates evolved to support a greater range of behaviors and physiological states, including states that we often associate with social engagement behaviors. How the “mammalian” autonomic nervous system fosters prosocial behaviors via a vagal brake The mammalian vagus (i.e., myelinated efferent pathways) functions as an active vagal brake (Porges et al., 1996) in which rapid inhibition and disinhibition of vagal tone to the heart can support behavioral mobilization or self-sooth and calm an individual. When the vagal tone to the pacemaker is high, the vagus acts as a restraint or brake limiting heart rate. When vagal tone to the 9 S.W. Porges 10 The Healing Power of Emotion (2009,in press) page pacemaker is low, there is little or no inhibition of the pacemaker. Due to vagal influences to the sino-atrial node (i.e., the heart's pacemaker), resting heart rate is substantially lower than the intrinsic rate of the pacemaker. Neurophysiologically the vagal brake provides a mechanism to support the metabolic requirements for mobilization and communication behaviors; functionally, the vagal brake, by modulating visceral state, enables the individual to rapidly engage and disengage with objects and other individuals and to promote self-soothing behaviors and calm behavioral states. Thus, withdrawal of the vagal brake is associated with adaptive states of mobilization and a reinstatement of the vagal brake with calm behavioral recovery. In mammals, the primary vagal inhibitory pathways occur through the myelinated vagus originating in the nucleus ambiguus. By transitory down-regulation of the cardioinhibitory vagal tone to the heart (i.e., removing the vagal brake), the mammal is capable of rapid increases in cardiac output without activating the sympathetic-adrenal system. This enables the ability to rapidly shift states from calm engagement to precautionary states of vagal withdrawal that rapidly increase cardiac output to support movements. But, unlike the sympathetic-adrenal strategy, which is slow to initiate and slower to dampen, reengaging the vagal brake instantaneously down regulates cardiac output to produce a calm physiological state (Vanhoutte and Levy, 1979). By withdrawing the vagal brake, rather than stimulating the sympathetic-adrenal system, mammals have an opportunity to rapidly increase metabolic output for immediate, but limited mobilization. If the duration and intensity of mobilization is increased, the sympathetic nervous system is activated. A withdrawal of the vagal brake will facilitate the recruitment of other neural mechanisms (e.g., excitation of sympathetic or the unmyelinated vagal pathways) and neural chemical mechanisms (e.g., stimulation of the HPA-axis) to regulate physiological state. Thus, consistent with the Polyvagal Theory, if the vagal brake is not functioning or will not serve the survival needs of the organism, then the phylogenetically “older” systems (e.g., the sympathetic-adrenal system or unmyelinated vagus originating in the dorsal motor nucleus of the vagus) will be recruited to regulate metabolic output to deal with environmental challenges. For example, if the vagal brake is not functioning, there is the potential for greater dependence on the sympathetic excitation of the cardiovascular system. This dependence on sympathetic excitation to regulate cardiac output may create health risks (e.g., hypertension) and lead to difficulties in modulating behavioral state (i.e., rage, panic, aggression). Consistent with assumptions of the Polyvagal Theory, the vagal brake contributes to the modulation of cardiac output by decreasing or increasing the inhibitory vagal control of the heart to influence rate and thereby adjust metabolic resources to support either mobilization or social engagement behaviors. The Social Engagement System As mammals evolved from more primitive vertebrates, a new circuit emerged to detect and to express signals of safety in the environment (e.g., to distinguish and to emit facial expressions and intonation of vocalizations) and to rapidly calm and turn off the defensive systems (i.e., via the myelinated vagus) to foster proximity and social behavior. This recent neural circuit can be conceptualized as a Social Engagement System. The Social Engagement System involves pathways traveling through several cranial nerves (i.e., V, VII, IX, X and XI) that regulate the expression, detection, and subjective experiences of affect and emotion. Neuroanatomically, this includes special visceral efferent pathways regulating the striated muscles of the face and head (i.e., special visceral efferent) and the myelinated vagal fibers regulating the heart and lungs (see Porges, 1998, 2001a, 2003). 10 S.W. Porges 11 The Healing Power of Emotion (2009,in press) page The Social Engagement System is an integrated system with both a somatomotor component regulating the striated muscles of the face and a visceromotor component regulating the heart via a myelinated vagus. The system is capable of dampening activation of the sympathetic nervous system and HPA-axis activity. By calming the viscera and regulating facial muscles, this system enables and promotes positive social interactions in safe contexts. The somatomotor component includes the neural structures involved in social and emotional behaviors. Special visceral efferent nerves innervate striated muscles, which regulate the structures derived during embryology from the ancient gill arches (Truex & Carpenter, 1969). The social engagement system has a control component in the cortex (i.e., upper motor neurons) that regulates brainstem nuclei (i.e., lower motor neurons) to control eyelid opening (e.g., looking), facial muscles (e.g., emotional expression), middle ear muscles (e.g., extracting human voice from background noise), muscles of mastication (e.g., ingestion), laryngeal and pharyngeal muscles (e.g., prosody of vocalizations), and head turning muscles (e.g., social gesture and orientation). Collectively, these muscles function as neural gatekeepers detecting and expressing features of safety (e.g., prosody, facial expression, head gestures, eye gaze) that cue others of intention and control social engagement with the environment. The phylogenic origin of the behaviors associated with the Social Engagement System is intertwined with the phylogeny of the autonomic nervous system. As the muscles of the face and head emerged as social engagement structures, a new component of the autonomic nervous system (i.e., a myelinated vagus) evolved that was regulated by nucleus ambiguus, a medullary nucleus ventral to the dorsal motor nucleus of the vagus. This convergence of neural mechanisms produced an integrated Social Engagement System with synergistic behavioral and visceral components as well as interactions among ingestion, state regulation and social engagement processes. As a cluster, difficulties in gaze, extraction of human voice, facial expression, head gesture and prosody are common features of individuals with autism and other psychiatric disorders in which the Social Engagement System is compromised. And thus, we infer from the functioning of the face and the prosody of the voice, difficulties in both social engagement behaviors and physiological state regulation. There are interneuronal connections between the source nuclei (i.e., lower motor neurons) of special visceral efferent pathways and the source nucleus of the myelinated vagus. These neurophysiological circuits provide an inhibitory pathway to slow heart rate and lower blood pressure, which, by actively reducing autonomic arousal, promote the calm states necessary to express social engagement behaviors and to support health, growth, and restoration. The brainstem source nuclei of this system are influenced by higher brain structures and by visceral afferents. Direct corticobulbar pathways reflect the influence of frontal areas of the cortex (i.e., upper motor neurons) on the medullary source nuclei of this system. Moreover, feedback through the afferent vagus (e.g., tractus solitarius) to medullary areas (e.g., nucleus of the solitary tract) influences both the source nuclei of this system and the forebrain areas that are assumed to be involved in several psychiatric disorders (e.g., Craig, 2005; Thayer & Lane, 2000). In addition, the anatomical structures involved in the Social Engagement System have neurophysiological interactions with the HPA axis, the social neuropeptides (e.g., oxytocin and vasopressin), and the immune system (for overview see Carter, 1998; Porges 2001b). Afferents from the target organs of the Social Engagement System, including the muscles of the face and head, provide potent afferent input to the source nuclei regulating both the visceral and 11 S.W. Porges 12 The Healing Power of Emotion (2009,in press) page somatic components of the Social Engagement System. Thus, activation of the behavioral component (e.g., listening, ingesting, looking) could trigger visceral changes that would support social engagement, while modulation of visceral state, depending on whether there is an increase or decrease in the influence of the myelinated vagal efferents on the sino-atrial node (i.e., increasing or decreasing the influence of the vagal brake) would either promote or impede social engagement behaviors. For example, stimulation of visceral states that would promote mobilization (i.e., fight or flight behaviors) would impede the ability to express social engagement behaviors. Relevant to psychiatric disorders are the specific deficits in both the somatomotor (e.g., poor gaze, low facial affect, lack of prosody, difficulties in mastication) and visceromotor (difficulties in autonomic regulation resulting in cardiopulmonary and digestive problems) components of the Social Engagement System. For example, clinicians and researchers have documented these deficits in individuals with autism. Thus deficits in the Social Engagement System would compromise spontaneous social behavior, social awareness, affect expressivity, prosody, and language development. In contrast, interventions that improve the neural regulation of the Social Engagement System, hypothetically would enhance spontaneous social behavior, state and affect regulation, reduce stereotypical behaviors, and improve vocal communication (i.e., including enhancing both prosody in expressive speech and the ability extract human voice from background sounds). This is more than a plausible hypothesis. We have not only demonstrated relations between vagal regulation of the heart and social engagement behaviors, but have demonstrated in preliminary studies that is possible to improve social engagement behaviors in autistic individuals by engaging the neural regulation of the social engagement system (i.e., stimulating the neural regulation of the middle ear muscles with exaggerated prosodic acoustic stimulation). Thus providing an empirical basis to understand the interpersonal social features, such as prosody and facial expressivity, that characterize individuals who effectively calm and sooth others. Disorders of the Social Engagement System: Maladaptive or adaptive behavioral strategies? Several psychiatric and behavioral disorders are characterized as having difficulties in establishing and maintaining relationships. Diagnostic features often include features associated with difficulties both in expressing social behavior and in reading social cues (i.e., social awareness). These features are observed in a variety of psychiatric diagnoses including autism, social anxiety, posttraumatic stress disorder, and reactive attachment disorder. From a psychopathology orientation, these clinical disorders have different etiologies and features. However, from a “polyvagal” perspective, they share a core component. This core component is characterized by a depressed social engagement system with the consequences of poor affect regulation, poor affect recognition, and poor physiological state regulation. Although a compromised Social Engagement System results in “maladaptive” social behavior, do these asocial behavioral strategies have “adaptive” features? The phylogeny of the vertebrate autonomic nervous system serves as a guide to understand these adaptive features. Through the lens of the Polyvagal Theory, the vertebrate autonomic nervous system follows three general stages of phylogenetic development. In the mammalian autonomic nervous system the structures and circuits representing each of the stages remain, but have been co-opted for various adaptive functions. The neural circuit associated with each stage supports a different category of behavior with the phylogenetically most recent innovation (i.e., the myelinated vagus) capable of supporting high levels of social engagement behavior. Since the neural regulation of the “new” mammalian myelinated vagus (i.e., ventral vagus) is integrated into the Social Engagement 12 S.W. Porges 13 The Healing Power of Emotion (2009,in press) page System, when the Social Engagement System is compromised, the effects are both behavioral and autonomic. The resultant changes in autonomic state compromise spontaneous social engagement behaviors and minimize states of calmness, but support a range of adaptive defensive behaviors. Specifically, the compromised Social Engagement System (see Figure 1) is associated, neurophysiologically, with a change in autonomic regulation characterized by a reduction in the influence of the myelinated vagus on the heart resulting in difficulties in behavioral state regulation and with a loss of neural regulation to the muscles of the face mediating the flat affective expression often observed in several clinical disorders. The removal of the regulatory influence of the myelinated vagus on the heart potentiates (i.e., disinhibits) the expression of the two phylogenetically older neural systems (i.e., sympathetic nervous system, unmyelinated vagus). These two older neural systems foster mobilization behaviors of fight and flight, via the sympathetic nervous system, or immobilization behaviors of death feigning, freezing and behavioral shut down via the unmyelinated vagus. Thus, withdrawal of the myelinated vagal circuit provides access to the more primitive adaptive defensive systems at a cost. If the removal is prolonged, there is an increased risk for both physical (e.g., risk for cardiovascular disorders) and mental (e.g., anxiety disorders, depression) illness as the protective anti-stress and self-soothing features of the myelinated vagus and the associated prosocial features of the Social Engagement System are lost. -----------------------------Insert figure 1 about here -----------------------------Neuroception: Contextual cueing of adaptive and maladaptive physiological states To effectively switch from defensive to social engagement strategies, the mammalian nervous system needs to perform two important adaptive tasks: 1) to assess risk, and 2) if the environment is perceived as safe, to inhibit the more primitive limbic structures that control fight, flight, or freeze behaviors. In other words, any intervention that has the potential for increasing an organism's experience of safety has the potential of recruiting the evolutionarily more advanced neural circuits that support the prosocial behaviors of the Social Engagement system. The nervous system, through the processing of sensory information from the environment and from the viscera, continuously evaluates risk. Since the neural evaluation of risk does not require conscious awareness and may involve subcortical limbic structures (e.g., Morris et al., 1999), the term neuroception (Porges, 2003) was introduced to emphasize a neural process, distinct from perception, that is capable of distinguishing environmental (and visceral) features that are safe, dangerous, or life threatening. In safe environments, autonomic state is adaptively regulated to dampen sympathetic activation and to protect the oxygen dependent central nervous system, and especially the cortex, from the metabolically conservative reactions of the dorsal vagal complex. However, how does the nervous system know when the environment is safe, dangerous, or life threatening and what neural mechanisms evaluate this risk? Neuroception might involve feature detectors involving the temporal cortex (see below), since the temporal cortex responds to familiar voice and faces and hand movements and can influence limbic reactivity. Thus, the neuroception of familiar individuals and individuals with appropriately prosodic voices and warm expressive faces translates into a social interaction promoting a sense of safety. In most individuals (i.e., without a psychiatric disorder or neuropathology) the nervous system evaluates risk and matches neurophysiological state with the actual risk of the environment. When the environment is appraised as being safe, the defensive limbic structures are inhibited enabling social engagement and calm visceral states to emerge. In 13 S.W. Porges 14 The Healing Power of Emotion (2009,in press) page contrast, some individuals experience a mismatch and the nervous system appraises the environment as being dangerous, even when it is safe. This mismatch results in physiological states that support fight, flight, or freeze behaviors, but not social engagement behaviors. According to the theory, social communication can be expressed efficiently through the Social Engagement System, only when these defensive circuits are inhibited. Neuroception represents a neural process that enables humans to engage in social behaviors by distinguishing safe from dangerous contexts. Neuroception is proposed as a plausible mechanism mediating both the expression and the disruption of positive social behavior, emotion regulation and visceral homeostasis. New technologies, such as fMRI, have identified specific neural structures that are involved in detecting risk. The temporal lobe is of particular interest in expanding the construct of neuroception and in identifying neural mechanisms that, by detecting and evaluating risk, modulate the expression of adaptive defensive behaviors and autonomic states. Functional imaging techniques document the involvement of the temporal cortex, fusiform gyrus and superior temporal sulcus, in the evaluation of biological movement and intention including the detection of features such as movements, vocalizations, and faces, which contribute to an individual being perceived as safe or trustworthy (Adolphs, 2002; Winston et al., 2002). Slight changes in these stimuli can be appraised as posing threat or alternatively signally endearment. Connectivity between these areas of the temporal cortex and the amygdala suggests a top-down control in the processing of facial features that could inhibit activity of the structures involved in the expression of defensive strategies (Pessoa et al., 2002). Neuroanatomical and neurophysiological research with animals provides additional information regarding the modulation and inhibition of defensive behaviors via well-defined connections among the amygdala, the periaqueductal gray (PAG), and the autonomic nervous system. The PAG is a heterogeneous midbrain structure that consists of gray matter surrounding the cerebral aqueduct that connects the third and fourth ventricles. Studies have identified areas of the PAG that are organized to regulate flight, fight, or freeze behaviors and the autonomic states that support these behaviors (Keay & Bandler, 2001). Stimulating rostrally within the lateral and dorsolateral PAG produces confrontational defensive behaviors (i.e., fight), while stimulating caudally within the lateral PAG and dorsolateral PAG produces escape behaviors (i.e., flight). Autonomic shifts such as increases in heart rate and blood pressure parallel these behaviors. In contrast, stimulation in the region of the PAG ventrolateral to the aqueduct (vlPAG) evokes a passive reaction of immobility, a drop in blood pressure, and a slowing of heart rate. Interestingly, excitation of the vlPAG evokes an opioid-mediated analgesia that might adaptively raise pain thresholds and promote the dissociative states that are frequently reported by trauma victims. In addition, there is evidence of a functional connection between the central nucleus of the amygdala and the vlPAG that both blunts the perception of pain (i.e.., antinociception) and promotes immobilization (Leite-Panissi, Coimbra, & Menescal-de-Oliveira, 2003). Consistent with the Polyvagal Theory, the vlPAG communicates with dorsal vagal complex (associated with immobilization in response to life threat), while the lPAG and dlPAG communicate with the sympathetic nervous system (associated with mobilization behaviors of fight/flight in response to danger). The detection of safety subdues the adaptive defensive systems dependent on limbic structures and enables the functioning of higher brain circuits related to frontal and temporal areas. This may provide a plausible model through which a neural detection of risk (i.e., neuroception) would modulate behavioral and physiological states to support adaptive behaviors in response to 14 S.W. Porges 15 The Healing Power of Emotion (2009,in press) page safe, dangerous, or life threatening environments. In the absence of threat, inhibitory projections from the fusiform gyrus and the superior temporal sulcus to the amygdala would be available to actively inhibit the limbic defense systems. This inhibition would provide an opportunity for social behavior to emerge. Thus, the appearance of a friend or mate would subdue the limbic activation with the biobehavioral consequences of allowing proximity, physical contact, and other social engagement behaviors. In contrast, during situations in which the appraisal of risk is high, the amygdala and various areas of the PAG are activated. The amygdala and PAG only share connections through the central nucleus of the amygdale (Rizvi et al., 1991). Based on the relative risk of the environment, both social engagement and defense behaviors may be interpreted as either adaptive or maladaptive. For example, the inhibition of defense systems by the Social Engagement System would be adaptive and appropriate only in a safe environment. From a clinical perspective it would be the inability to inhibit defense systems in safe environments (e.g., Anxiety Disorders, PTSD, Reactive Attachment Disorder) or the inability to activate defense systems in risk environments (e.g., Williams Syndrome, a genetic disorder with a behavioral repertoire characterized by engaging without detecting or respecting the emotional state of others) that might contribute to the defining features of psychopathology. Thus, an invalid neuroception of safety or danger might contribute to maladaptive physiological reactivity and the expression of the defensive behaviors associated with specific psychiatric disorders that include in their diagnostic criteria, a social deficit (e.g., autism, social anxiety, Williams Syndrome) or fear (e.g., various phobias, Obsessive-Compulsive Disorder) (Leckman et al., 1997). However, in most individuals neuroception accurately reflects risk and there is a consistency between the cognitive awareness of risk and the visceral response to risk. The features of risk in the environment do not solely drive neuroception. Afferent feedback from the viscera provides a major mediator of the accessibility of prosocial circuits associated with social engagement behaviors. For example, the Polyvagal Theory predicts that states of mobilization would compromise our ability to detect positive social cues. Functionally, visceral states color our perception of objects and others. Thus, the same features of a person engaging another may result in range of outcomes, depending on the physiological state of the target individual. If the person being engaged is in a state in which the social engagement system is easily accessible, the reciprocal prosocial interactions are likely to occur. However, if the individual is in a state of mobilization, the same engaging response might be responded to with the asocial features of withdrawal or aggression. In such a state, it might be very difficult to dampen the mobilization circuit and enable the Social Engagement System comes back on line. The insula may be involved in the mediation of neuroception, since it has been proposed as a brain structure involved in conveying the diffuse feedback from the viscera into cognitive awareness. Functional imaging experiments have demonstrated that the insula has an important role in pain experience and the experience of several emotions, including anger, fear, disgust, happiness and sadness. Critchley et al. (2004) propose that internal body states are represented in the insula and contribute to subjective feeling states and have demonstrated that activity in the insula correlated with interoceptive accuracy. Co-opting the immobilization defense system for reproductive behaviors, nursing, and the formation of social bonds. Immobilization as a defense system is phylogenetically old and is associated with reduced metabolic demands and increased pain threshold. In reptiles, with a larger tolerance for reductions 15 S.W. Porges 16 The Healing Power of Emotion (2009,in press) page in oxygen, immobilization is a very effective defense strategy. In contrast, since mammals have a great need for oxygen, the inhibition of movement coupled with a shift in autonomic state to support the immobilization behavior (i.e., apnea and bradycardia) can be lethal (Hofer, 1970; Richter, 1957) such that death feigning can lead to death. In humans, fainting or dissociating in anticipation of death or painful injury reflects a less extreme form of this response. However, several aspects of mammalian social behavior require immobilization, but do so in the absence of life threat. In these contexts an immobilization without fear is required. Immobilization without fear is accomplished by co-opting the structures that regulate immobilization in response to life threat to serve a broad range of social needs including reproduction, nursing, and pair bonding. The area of the PAG that coordinates freezing behavior, primitive immobilization defense system has been modified in mammals to serve their intimate social needs. In addition, it has been reported that the vlPAG is rich in receptors for oxytocin, a neuropeptide associated with parturition, nursing, and the establishment of pair bonds (Carter, 1998; Insel & Young 2001). Through the process of evolution the immobilization circuit has been modified to enable prosocial behaviors associated with reproduction and nursing. This circuit enables humans to sleep safely with each other or for a baby to safely nurse on the breast of the mother. Overlapping with the area of the PAG that organizes immobility (i.e., vlPAG) are areas that when stimulated produce lordosis and kyphosis. The lordosis reflex is a hormone-dependent behavior displayed by female rodents and other mammalian species during mating. In most mammals lordosis involves the female immobilizing in a crouching posture with her hind end available to the male for copulation. Neural tracing studies have demonstrated that the vlPAG is part of the neural circuit involved in regulating lordosis (Daniels, Miselis, & Flanagan-Cato, 1999). Kyphosis is an upright arched back posture that is accompanied by inhibition of limb movements. Kyphosis is stimulated by nipple attachment and provides an opportunity for the dam to feed simultaneously a large litter. When dams initiate a nursing bout, their behavioral state shifts immediately from high activity to immobility (Stern, 1997). When the caudal portion of the vlPAG is lesioned there are important consequences: 1) kyphotic nursing decreases, 2) litter weight gains decrease, and 3) the lesioned rats are more aggressive and more frequently attack strange males (Lonstein & Stern, 1998). Humans, similar to other mammals, have co-opted this ancient immobilization circuit to support nursing and reproductive behaviors. Co-opting the mobilization defense system for play Often the playful “rough and tumble” behaviors observed in mammals are interpreted as preliminary exercises to develop adaptive defensive and aggressive behaviors. However, play is also inherently motivating and provides a unique and positive experience (Panksepp, 1998). Play, at least rough and tumble play, is characterized by mobilization. Thus, play shares with the defensive fight-flight behaviors a neurophysiological substrate that functionally increases metabolic output by increasing sympathetic excitation. Concurrent with the sympathetic excitation is a withdrawal of the myelinated vagal pathways that characterize the vagal brake. Just as the primitive mechanisms mediating immobilization in response to life-threat can be co-opted to support loving and nutrient processes, so can mobilization mechanisms be involved occur to facilitate both defensive “flight-fight” behaviors and the pleasurable “play.” How is play distinguished from aggressive behavior? More importantly, are there “neuroceptive” processes that either dampen or potentiate aggressive retaliation? If we observe 16 S.W. Porges 17 The Healing Power of Emotion (2009,in press) page play, we can reliably observe cues lead to either aggressive or calming. Frequently play leads to acts that are painful and potentially aggressive. For example, often a playmate is injured. This may occur with various mammalian species. When puppies play, they may bite too hard and elicit a painful cry in the playmate. When a human is playing with a dog, the dog might be accidentally hit in a vulnerable and tender place like the nose. When humans play a sport such as basketball, an individual may be hit with an elbow to the face. How are these situations diffused? What processes enable anger to be contained and play to be resumed? Access to the Social Engagement System can transform potential aggression to play. The Social Engagement System cues others that the “intentionality” of the behavior is benign. For example, a fight is likely to occur, if the individual who accidentally hits another in the face while playing basketball walks away without diffusing the tension through a face-to-face expression of concern. Similarly, play will not continue, if puppies playing do not make face-to-face engagements after an accidental but hurtful bite. Consistent with the importance of the Social Engagement System in the process of play, autism is associated with a lack of non-interactive (i.e., parallel) play. Thus, access to the Social Engagement is critical in defining mobilization as play and not aggression. Team sports, which are prevalent in our culture, involve mobilization strategies that require face-to-face interactions to signal intentionality and share the common feature of integrating features of the Social Engagement System with mobilization. Jogging and other forms of exercise also result in a physiological state similar to team sports or rough and tumble play. However, unlike exercise, a “polyvagal” definition of play requires reciprocal interactions and a constant awareness of the actions of others. Play is different than fight-flight behaviors. Although fight-flight behaviors often require an awareness of others, fightflight behaviors do not require reciprocal interactions and an ability to restrain mobilizations. Play recruits another circuit that enables aggressive and defensive behaviors to be contained. The rapid recruitment of the Social Engagement System results in an immediate face-to-face evaluation of whether there is intentionality in the event that provoked the painful response. Areas of the cortex, such as superior temporal sulcus, provide a plausible location for this neuroceptive process. The superior temporal sulcus has been proposed to be an area of the brain that evaluates biological movement and intentionality. Thus, familiar voices, calming gestures, and appropriate facial expression can rapidly diffuse a possible physical conflict. Even the dogs that whimpers after being hit on the nose or bit on the leg while playing will rapidly make a face-to-face engagement and wait for a gesture that will provide the reassurance that the event was not intentional. How does the Social Engagement System calm us down and keep us from expressing inappropriate aggressive acts? First, there are inhibitory pathways from the temporal cortex that dampen the limbic reactivity associated with defensive behaviors. Second, as Gellhorn (see above) noted almost 50 years ago, facial muscle activity influences the brain structures that regulate visceral state. This is frequently observed in mammals of all ages from the very young infants who use sucking behaviors to calm to older individuals who use conversation, listening, smiling, and ingesting to calm. Consistent with and in contrast to these strategies of defusing conflict, walking away or turning the head away from the conflict can trigger a violent reaction. By investigating the unique physiological mechanisms involved in play, we uncover the unique properties of reciprocal interactions that may define play and distinguish it from exercise and other solitary behaviors. Play requires turn taking in expressive motor movements and reciprocal receptive inhibition of activity. This is also observed in talking and listening, in throwing and catching, and in hiding and seeking. When there is mutual activity and contact, such as in 17 S.W. Porges 18 The Healing Power of Emotion (2009,in press) page rough and tumble play, there are more opportunities for cues to be mistaken and aggressive behaviors to unfold. However, if face-to-face engagement occurs rapidly with the appropriate features of concern and empathy, then the physiological state that was driven by the physical contact is evaluated for intentionality and diffused with the appropriate cues exchanged between two Social Engagement Systems involved in the face-to-face exchange. Although play may share some of the neural mechanisms involved in fight-flight behaviors, unlike solitary exercise, play requires dynamic neural regulation of state to insure safe interactions. Thus, both sympathetic activation to increase metabolic output to support motor activity and the vagal brake to restrain mobilization and to support the function of the Social Engagement System are recruited to maintain a mutual playful activity. Another adaptive process involves the co-activation of sympathetic excitatory and vagal inhibitory processes. This process is associated with sexual arousal, another vulnerable state that evolutionarily requires face-to-face interactions to evaluate intentionality of physical contact to determine whether the behaviors are caring or hurtful. Summary statements The Polyvagal Theory is an attempt to reorganize our conceptualization of the autonomic nervous system with a focus on the specific neural circuits involved in regulating visceral organs for specific adaptive functions including the domains of affect, emotions, and goal directed behaviors. The theory identifies specific variables that can be used to dynamically evaluate the changing neural regulation of specific adaptive circuits. Implicit in the theoretical model are four prominent features that impact directly on the development of testable hypotheses: 1) the role specific brain structures and neural circuits have in regulating autonomic state, 2) the justification of developing methods that can distinguish and track the dynamic vagal output to target organs through the myelinated vagus originating in the nucleus ambiguus and the unmyelinated vagus originating in the dorsal motor nucleus, 3) the role visceral afferents and sensory feature detectors have on the switching among the neural circuits regulating autonomic state, and 4) the relation between the regulation of visceral organs and the regulation of the striated muscles of the face and head involved in social engagement behaviors including affect recognition and emotional expression. The Polyvagal Theory suggests that affective or emotional states are dependent upon lower brain regulation of the visceral state and the important visceral, tactile, and nocioceptive cues that travel between the brain and the periphery. Through the lens of the Polyvagal Theory, specific bodily states foster different domains of behavior. Specifically, the neural regulation of five physiological states has been described and each state has been linked with a specific biologically based behavioral repertoire. 1) Social engagement: A state dependent on a well-defined social engagement system. This system promotes positive social interactions, reduces psychological distance, and promotes a sense of safety between people. 2) Mobilization- fight/flight: This state supports fight and flight behaviors and requires an increase in metabolic output. 3) Play: A blend of the above. Play is a hybrid state requiring features from both states of mobilization and social engagement. 4) Immobilization- Life threat: This state is associated with life threat and is characterized by a reduction of metabolic output and shutdown behaviors. This primitive neural circuit works fine for reptiles but is potentially lethal in mammals. 5) Immobilization without fear: This state is associated with prosocial and positive states that require a reduction of movement without the massive reduction of metabolic resources. 18 S.W. Porges 19 The Healing Power of Emotion (2009,in press) page This circuit recruits pathways from the immobilization circuit and is used during nursing, childbirth, and reproductive behaviors and digestive and restorative processes. Functionally, these five states color our perception of objects and others. Thus, the same features of a person engaging another may result in range of outcomes, if the target individual is in a different physiological state. If the person being engaged is in a state in which the social engagement system is easily accessible, the reciprocal prosocial interactions are likely to occur. If the individual is in a state of mobilization, the same engaging response might be responded to with the asocial features of withdrawal or aggression. This Stimulus-Organism-Response model is reminiscent of Woodworth (1928), who postulated an S-O-R model with an active organism intervening between stimulus and response. In the Woodworth model processes internal to the organism mediated the effects of stimuli on behavior. Within the Polyvagal Theory, neuroception is a S-O-R model. Within this context, autonomic state is an intervening process that contributes to the transformation from the external physical stimulus to the complex internal cognitive-affective processes that determine the quality of the interpersonal interaction. The five states described above provide a good fit with the underlying physiological states required to successfully express the seven neural-based systems described by Panksepp (1998). Moreover, the Polyvagal Perspective, with its emphasis on phylogenetic shifts in visceral regulation, provides a unique insight into the use of psychological constructs. For example, the Polyvagal Theory will lead to three different visceral phenotypes for the emotion of fear. One type is characterized by mobilization strategies consistent with the features of fight-flight behaviors. A second type is characterized by immobilization (e.g., death feigning), a biobehavioral state that, due to metabolic depression, can potentially be lethal for a mammal. In humans this might be observed as fainting, defecating, and/or dissociating. A third type is more cognitive and involves a transitory depression of the Social Engagement system as a precautionary response to evaluate intentionality behaviors. If behavior is detected as dangerous, then the sympathetic nervous system is activated to support the fight/flight mobilization behaviors. All three are “fear” responses, but they have different behavioral topographies and different underlying neurophysiological substrates. Thus, the understanding of affective experiences and the strategy of organizing of these experiences into psychological constructs such as “emotions” may be informed by understanding the covariation between the specific phylogenetic shifts in the neural regulation of the viscera and the adaptive nature of these various affective states in phylogenetically older vertebrates Concluding comments To optimize strategies studying the bridge between nervous system function and both clinical disorders and affective experiences, affective neuroscience will need to incorporate methodologies and to test hypotheses dependent on our expanding knowledge of neurophysiology and the central structures involved in both appraisal of context (i.e., neuroception) and neural regulation of visceral state. These questions have motivated previous (e.g., Cannon, Darwin, James, Gellhorn, Hess) and contemporary researchers (e.g., Critchly, 2005, Ekman, Levenson, Friesen, 1983; Thayer & Lane, 2000) attempting to bridge the gap between visceral states and the subjective labels of affective experiences (i.e., emotions). To close this gap, new methodologies are necessary that are capable of evaluating dynamic changes in and interactions among various physiological (e.g., respiration, heart rate, blood pressure, vasomotor tone, and motor activity) variables in a changing context. In response to these needs, the Polyvagal Theory was developed. 19 S.W. Porges 20 The Healing Power of Emotion (2009,in press) page The Polyvagal Theory provides a perspective to demystify features of clinical disorders. The theory provides principles to organize previously assumed disparate symptoms observed in several psychiatric disorders (i.e., a compromise in the function of the Social Engagement System). Moreover, by explaining features of disorders from an adaptive perspective, interventions may be designed that trigger the neural circuits that will promote spontaneous social engagement behaviors and dampen the expression of defensive strategies that disrupt social interactions. 20 S.W. Porges 21 The Healing Power of Emotion (2009,in press) page References Adolphs R. (2002). Trust in the brain. Nature Neuroscience, 5, 192-193. Berlyne DE. (1960). Conflict, Arousal, and Curiosity. New York: McGraw-Hill. Cannon WB. (1929). Organization for physiological homeostasis. Physiological Reviews, 9, 399431. Carter CS. (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23, 779-818. Craig AD. (2005). Forebrain emotional asymmetry: a neuroanatomical basis? Trends in Cognitive Sciences, 9, 566-571. Critchley HD. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology, 493, 154-166. Critchley HD. Wiens S, Rotshtein P, Ohman A, & Dolan RJ. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189-195. Damasio A. (1999). The feeling of what happens. New York” Harcourt, Brace. Daniels D, Miselis RR, & Flanagan-Cat, LM. (1999). Central neuronal circuit innervating the lordosis-producing muscles defined by transneuronal transport of pseudorabies virus. Journal of Neuroscience, 19, 2823-2833. Darrow CW. (1943) Physiological and clinical tests of autonomic function and autonomic balance. Physiological Reviews, 23: 1-36. Darwin C. (1872) The Expression of Emotions in Man and Animals. New York: D. Appleton. Ekman P, Levenson RW, Friesen WV. (1983) Autonomic nervous system activity distinguishes among emotions. Science, 16;221:1208-10. Gellhorn E. (1964). Motion and emotion: the role of proprioception in the physiology and pathology of the emotions. Psychological Review, 71, 457-472. Gray JA. (1971) The Psychology of Fear and Stress. New York: McGraw Hill. Hess (1949) Hess WR (1954) Diencephalon: Autonomic and Extrapyramidal Functions. New York: Grune and Stratton. Hofer MA. (1970). Cardiac respiratory function during sudden prolonged immobility in wild rodents. Psychosomatic Medicine, 32, 633-647. Insel TR & Young LJ. (2001). The neurobiology of attachment. Nature Reviews Neuroscience. 2,129-136. Keay KA, & Bandler R. (2001). Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and Biobehavioral Reviews. 25:669-678. Langley JN (1921). The Autonomic Nervous System . Cambridge, England: Heffer & Sons Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, & Pauls DL. Symptoms of obsessive-compulsive disorder American Journal of Psychiatry, 154,911-917. Leite-Panissi CR, Coimbra NC & Menescal-de-Oliveira L. (2003). The cholinergic stimulation of the central amygdala modifying the tonic immobility response and antinociception in guinea pigs depends on the ventrolateral periaqueductal gray. Brain Research Bulletin. 60, 167-178. Lonstein JS & Stern JM. (1998). Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Research, 804,21-35. Morris JL and Nilsson S (1994) The circulatory system. In Nilsson S and Holmgren S (eds.) Comparative Physiology and Evolution of the Autonomic Nervous System, pp. 193-246. Switzerland: Harwood Academic Publishers. Morris JS, Ohman A, & Dolan RJ. (1999). A subcortical pathway to the right amygdale mediating “unseen” fear. Proceedings of the National Academy of Sciences, 96, 1680-1685.. Panksepp J. (1998). Affective Neuroscience. New York” Oxford University Press. 21 S.W. Porges 22 The Healing Power of Emotion (2009,in press) page Pavlov IP. (1927) Conditioned Reflexes. London: Oxford University Press. Pessoa L, McKenna M., Gutierrez, E, & Ungerleider LG. (2002). Neuroprocessing of emotional faces requires attention. Proceedings of the National Academy of Sciences (USA), 99, 11,458-11,463. Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology, 32: 301-318. Porges SW (1997) Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. In Carter CS, Kirkpatrick B and Lederhendler II (eds.) The Integrative Neurobiology of Affiliation. Annals of the New York Academy of Sciences 807: 62-77. Porges SW. (1998) Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology, 23: 837-861. Porges SW. (2001a). The Polyvagal Theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42, 123-146. Porges SW. (2001b). Is there a major stress system at the periphery other than the adrenals? In: D.M. Broom (ed.) Dahlem Workshop on Coping with Challenge: Welfare in Animals including Humans, pp. 135-149. Porges SW. (2003). Social engagement and attachment: A phylogenetic perspective. Roots of Mental Illness in Children, Annals of the New York Academy of Sciences, 1008, 31-47. Porges SW. (2007). The polyvagal perspective. Biological Psychology, 74:116-43. Porges SW, Doussard-Roosevelt JA, Portales AL, and Greenspan SI (1996) Infant regulation of the vagal "brake" predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology, 29: 697-712. Richter CP. (1957). On the phenomenon of sudden death in animals and man. Psychosomatic. Medicine, 19,191-198. Rizvi TA, Ennis M, Behbehani MM, & Shipley MT. (1991). Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. Journal of Comparative Neurology, 303,121-131. Schore A. (1994). Affect regulation and the origin of the self: The neurobiology of emotional development. Hillsdale: Erlbaum. Schore A. (2003). Affect dysregulation and disorders of the self. New York: Norton. Selye H. (1936) A syndrome produced by diverse nocuous agents. Nature, 138: 32. Selye H. (1956) The Stress of Life. New York: McGraw-Hill Siegel D. (2007). The Mindful Brain. New York: Norton. Stern JM. (1997). Offspring-induced nurturance: animal-human parallels. Developmental Psychobiology, 31, 19-37. Thayer JF, & Lane RD. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201-216. Truex RC & Carpenter MB (1969). Human Neuroanatomy. 6th edition. Williams and Wilkins, Baltimore, MD. Vanhoutte PM & Levy MN (1979) Cholinergic inhibition of adrenergic neurotransmission in the cardiovascular system. In: Brooks CM, Koizumi K, and Sato A. Integrative Functions of the Autonomic Nervous System, pp. 159-176. Tokyo: The University of Tokyo Press. Winston JS, Strange, BA, O’Doherty J, & Dolan, RJ. (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience, 5,277-283. Woodworth RS. (1928). Dynamic psychology. In C. Murchison (Ed.), Psychologies of 1925. Worcester, MA: Clark University Press. 22 S.W. Porges 23 The Healing Power of Emotion (2009,in press) page Figure Captions Figure 1. The Social Engagement System. Social communication is determined by the cortical regulation of medullary nuclei via corticobulbar pathways. The Social Engagement System consists of a somatomotor compnent (i.e., special visceral effernt pathways that regulate the striated muscles of the face and head) and a visceromotor component (i.e., the myelinated vagus that regulates the heart and bronchi). Solid blocks indicate the somatomotor component. Dashed blocks indicate the visceromotor component. i In 1949 W. Hess was awarded the Nobel Prize in Physiology or Medicine (see Hess, 1954). His Nobel lecture was entitled “The Central Control of Activity of Internal Organs.” In his lecture Hess acknowledged the importance of the prevailing model of the autonomic nervous system, which emphasized the paired antagonistic innervations of the internal organs and the definition of sympathetic and parasympathetic functions. However, he went well beyond this conceptualization to emphasize the importance of central structures in the regulation of visceral state by describing his studies that demonstrate the influence of the hypothalamus on the autonomic nervous system. By emphasizing the central mechanisms that mediate the dynamic regulation of peripheral organs, Hess anticipated the need for methodologies and technologies to continuously monitor the neural circuits involving both defined brain structures and peripheral nerves in the regulation of visceral function and state and to move from the prevailing conceptualization of the autonomic nervous system as a peripheral system. Hess’s lecture: 1) emphasized the importance of feedback circuits linking peripheral organs to brain structures and the bidirectionality of these feedback circuits, and 2) acknowledged that, although much can be learned about neural structures and functions via traditional experimental paradigms (e.g., neural blockade, surgery, electrical stimulation), dynamic feedback circuits, i.e., the moment-to-moment dynamic shifts in the system, cannot be adequately studied through these paradigms. 23