Disproportionation and comproportionation reactions of resistive
advertisement

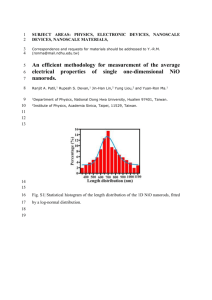
APPLIED PHYSICS LETTERS 98, 153513 共2011兲 Disproportionation and comproportionation reactions of resistive switching in polycrystalline NiOx films F. S.-S. Chien,1,a兲 Y. T. Wu,1 G. L. Lai,1 and Y. H. Lai2 1 Department of Physics, Tunghai University, Taichung 407, Taiwan Department of Chemistry, Tunghai University, Taichung 407, Taiwan 2 共Received 19 January 2011; accepted 12 March 2011; published online 14 April 2011兲 NiOx thin film exhibited excellent bistable unipolar resistive switching 共RS兲, which has a high potential in nanoscale nonvolatile-memory applications. The underlying reaction of RS in NiOx is still in debate. We studied the chemical bonding states of Ni 2p and O 1s at high/low resistance spots using focused x-ray photoelectron spectroscopy. The disproportionation and comproportionation reactions of 3NiO↔ Ni+ Ni2O3 accounted for the RS of NiOx. The calculated Gibbs energy of the reaction interpreted its reversibility in thermodynamics. The expansion and contraction of the filaments with switching were observed by conducting atomic force microscopy. © 2011 American Institute of Physics. 关doi:10.1063/1.3574908兴 a兲 Electronic mail: fsschien@thu.edu.tw. 0003-6951/2011/98共15兲/153513/3/$30.00 Pt 10 40 10 -1 10 -3 10 -5 10 -7 60 Pt NiO (311) 2 Pt Pt Si NiO (220) 3 10 Pt (a) NiO (200) 4 10 NiO (111) XRD intensity (counts) NiO film in ON and OFF states, both ON and OFF spots were analyzed by focused XPS 共ULVAC-PHI, PHI 500 VersaProbe兲. The scanning focused x-ray beam was 10 m in diameter and the spectra of photoemission 共PE兲 electrons were obtained at specific micro areas. PE spectra of Ni 2p3/2 and O 1s in the as-deposited film, and the ON and OFF spots are shown in Fig. 2. The main peaks of Ni 2p3/2 spectra consist of the components Ni0 共Ni, 852.5 eV兲, Ni2+ 共NiO, 854 eV兲, Ni2+ 关Ni共OH兲2, 855.7 eV兴, Ni+3 共Ni2O3, 857 eV兲 and the satellite peaks of Ni, NiO, and Ni2O3.8 The spectra of O 1s have the components O2− 共NiO, 529.6 eV兲 and O2− 共Ni2O3, 531.5 eV兲. It is hard to resolve the O2− of Ni共OH兲2 from the O2− of NiO, because their binding energies are too close. In the as- Current (A) Bistable resistive switching 共RS兲 in transition metal oxide 共TMO兲 thin films is an interesting phenomenon for its giant resistance ratio 共⬃104兲 of low conductivity state to high state.1 Also the switching demonstrates high potential for applications in high-speed and high-density nonvolatile memories of next generations.2 The generally accepted mechanism for RS of TMOs is the formation/rupture of microscopic conductive channels 共also called filaments兲 in the oxide matrix.3 For example, metallic Ni filaments are formed in NiO films due to the electrical joule heat and/or electrical field.4 Analysis with transmission electron microscopy exhibits the existence of Ni filaments at the defective area 共e.g., the grain boundary兲 in NiO films for RS and the migration of oxygen is proposed to be strongly correlated with the formation of Ni filaments.3,5,6 The formation/rupture of Ni filaments manifests some reduction/oxidation reactions occurring, but the chemical reactions responsible for the phenomenon are unclear. To determine the reaction related to RS, we characterized the chemical bonding states of the high conductivity state 共ON state兲 and low conductivity state 共OFF state兲 on NiO films by focused x-ray photoelectron spectroscopy 共XPS兲.5,7 It is found that the disproportionation and comproportionation reactions 共3NiO↔ Ni+ Ni2O3兲 account for the reversible and repeatable switching. RS was also performed by scanning probes and the dynamics of metallic filaments with RS was studied in situ by conductive atomic force microscopy 共CAFM兲. The NiO film was deposited on the Ti/Pt bottom electrode by reactive dc magnetron sputtering with a Ni target in a low oxygen environment. The NiO film was subjected to thermal annealing at 450 ° C in air for 1 h. Figure 1共a兲 shows the grazing incident x-ray diffraction 共XRD兲 pattern, indicating the NiO film was polycrystalline with fcc structure. To perform the RS of the NiO film, a tungsten probe was used as the top electrode applied with a negative bias. Figure 1共b兲 is the I-V curves of unipolar RS, showing the FORMING, RESET, and SET processes occurred at ⫺3.1 V, ⫺0.9 V, and ⫺1.6 V, respectively. The diameter of the RS spots was about 20 m. To understand the chemical bonding states of the 2 (degree) 80 (b) Reset Set Forming 0 -1 -2 Voltage (V) -3 FIG. 1. 共Color online兲 共a兲 Grazing-incident XRD pattern of the NiO thin film and 共b兲 I-V curves of RS of the NiO film with a tungsten probe as the top electrode. 98, 153513-1 © 2011 American Institute of Physics Downloaded 03 May 2011 to 129.234.252.65. Redistribution subject to AIP license or copyright; see http://apl.aip.org/about/rights_and_permissions Appl. Phys. Lett. 98, 153513 共2011兲 Chien et al. (a) 1L2+ 1L S 1L2 1L2 PE Intensity (a.u.) 1L (c) (b) 1L2 250 2 V 1L2 'G (kJ/mol) 153513-2 (d) 200 150 100 50 0 0 (e) (f) 500 1000 Temperature 1500 q& FIG. 3. 共Color online兲 Calculated Gibbs energy of the disproportionation reaction 3NiO→ Ni+ Ni2O3 as a function of the reaction temperature. 865 860 855 850 534 532 530 528 526 Binding Energy (eV) Binding Energy (eV) FIG. 2. 共Color online兲 PE spectra of Ni 2p3/2 and O 1s taken in the original NiO film 共a兲 and 共b兲, the ON spot 共c兲 and 共d兲, and the OFF spot 共e兲 and 共f兲. deposited film 关Figs. 2共a兲 and 2共b兲兴, NiO was dominant and small amounts of higher-oxide-state Ni2O3 were observed in both Ni and O spectra. Small traces of metallic Ni appeared in the Ni spectra. Typically the as-deposited NiO is Nideficient p-type semiconductor, but metallic Ni defect still exists as the film is prepared in a low oxygen environment.9 Also Ni共OH兲2 was formed due to the exposure to humid ambience. In both Ni and O spectra at the ON spot 关Figs. 2共c兲 and 2共d兲兴, the component of NiO decreased, while Ni2O3 increased. In addition, the metallic Ni increased significantly 关Fig. 2共c兲兴. The increased metallic Ni is associated with the conductive filament. The formation of a Ni filament is attributed to the migration of oxygen induced by the electric field and joule heat of the electric current. The doubly positive 2+ 兲 and two electrons were generated oxygen vacancies 共VO 共O2− → 2e− + O兲, so the nearby Ni2+ was reduced to neutral Ni 共Ni2+ + 2e− → Ni兲.6 Several types of evidence indicate that the metallic Ni filaments precipitate along the NiO grain boundary.5 It is worth noting that the Ni2O3 components in both Ni 2p and O 1s spectra increased after the initial FORMING process. We suggest the oxygen atoms diffused into the surrounding NiO grains to produce Ni2O3. Therefore the formation of filaments involves the forward disproportionation reaction, NiO → Ni + O 2NiO + O → Ni2O3 , 3NiO → Ni + Ni2O3 共1兲 in which NiO was simultaneously reduced and oxidized so as to form two products with different oxidation states 共Ni and Ni2O3兲. In the RESET, the products of the forward reaction became the reactants in the reverse comproportionation reaction, Ni + Ni2O3 → 3NiO. 共2兲 Therefore RS is associated with the self structural reorganization between nickel oxides. Such a self reorganization is also revealed in the RS reactions of other TMOs, where TiO↔ Ti4O7 takes place.10,11 It is well known that bulk NiO is oxidized to Ni2O3 by absorbing oxygen at an elevated temperature of 400 ° C and then Ni2O3 is reduced to NiO at 600 ° C.12 The local temperature of the filament in the RESET process is estimated to be over 450 ° C with the steady state temperature model,4 which should be sufficient to induce the switching reactions in nanoscale. The Gibbs energy 共⌬G ° = ⌬H ° −T⌬S°兲 of the disproportionation reaction with the initial temperature 25 ° C at standard pressure was calculated as a function of the reaction temperature T, shown in Fig. 3. The thermodynamic coefficients to derive ⌬H° 共enthalpy兲 and ⌬S° 共entropy兲 are available in Ref. 13. We believe the Gibbs energy for RS that occurred in the nanoscale volume around the grain boundary should be much lower than the calculated values, but at the nanoscale the dependence of Gibbs energy on temperature should be similar to the calculated result of bulk NiO. ⌬G° is positive/negative for the forward/reverse reaction below some 2000 ° C. The forward reaction does not occur spontaneously at room temperature for positive ⌬G°, unless enough energy is provided. As there are metallic Ni and Ni2O3 produced by the forward reaction, the reverse reaction with negative ⌬G° precedes the forward reaction as the joule heat is provided. Such a reaction pair can cyclically repeat as the size of the Ni filaments is within the diffusion length of oxygen. The local conductivity of the RS spots was analyzed by CAFM 共Agilent 5500 AFM兲. The metallic Cr/Pt coated scanning probe replaced the tungsten probe to produce the ON and OFF spots on the NiO film. The local RS is shown in Fig. 4共a兲. The RS behavior is the same as that made by conventional tungsten probes. Figure 4共b兲 is the image of current mapping at the ON and OFF spots and the crosssection profile of the current across the spots. The bright areas correspond to the RS areas with metallic Ni filaments. Both ON and OFF spots have a higher conductivity than the as-deposited background, which agrees with the I-V characteristics. Apparently, the current at the ON spots 共ION兲 is higher than the current at the OFF 共IOFF兲. The ratio of ION and IOFF is only five, not comparable with the ratio from the I-V characteristics 共103 – 104兲 at Fig. 4共a兲, because the conductivity of the probe-surface junction during scanning can be affected by the contact force, scan speed and surface contamination. A similar current ratio 共20–30兲 from CAFM mapping of a NiO film has been reported.5 It is worth noting that the lateral size of the spots varies with the ON/OFF states. From the current mapping, the mean lateral size of the spots Downloaded 03 May 2011 to 129.234.252.65. Redistribution subject to AIP license or copyright; see http://apl.aip.org/about/rights_and_permissions 153513-3 (a) Current (A) Appl. Phys. Lett. 98, 153513 共2011兲 Chien et al. 10 -6 10 -8 10 Reset Set Forming -10 0.0 -2.0 -4.0 Voltage (V) -6.0 Current (nA) (b) 20 This work was supported by the National Science Council, Taiwan 共Grant Nos. NSC 99-2112-M-029-005-MY3 and NSC 98-2113-M-029-006-MY2兲. The authors would like to thank Mr. S. M. Cheng of ITRI for his help in XRD measurement. on off 0 0.0 0.5 1.0 Position (Pm) 1.5 Current (nA) 30 15 0.4 Position (Pm) R. Waser, R. Dittman, G. Stikov, and K. Szot, Adv. Mater. 共Weinheim, Ger.兲 21, 2632 共2009兲. 2 I. G. Baek, M. S. Lee, S. Seo, M. J. Lee, D. H. Seo, D.-S. Suh, J. C. Park, S. O. Park, H. S. Kim, I. K. Yoo, U.-I. Chung, and J. T. Moon, Tech. Dig. - Int. Electron Devices Meet. 2004, 587. 3 G.-S. Park, X.-S. Li, D.-C. Kim, R.-J. Jung, M. J. Lee, and S. Seo, Appl. Phys. Lett. 91, 222103 共2007兲; H. Shima, F. Takano, H. Akinaga, Y. Tamai, I. H. Inoue, and H. Takagi, ibid. 91, 012901 共2007兲. 4 D. C. Kim, S. Seo, E. Ahn, D.-S. Suh, M. J. Lee, B.-H. Park, I. K. Yoo, I. G. Baek, H.-J. Kim, E. K. Yim, J. E. Lee, S. O. Park, H. S. Kim, U.-I. Chung, J. T. Moon, and B. I. Ryu, Appl. Phys. Lett. 88, 202102 共2006兲. 5 M.-J. Lee, S. Han, S. H. Jeon, B. H. Park, B. S. Kang, S.-E. Ahn, K. H. Kim, C. B. Lee, C. J. Kim, I.-K. Yoo, D. H. Seo, X.-S. Li, J.-B. Park, J.-H. Lee, and Y. Park, Nano Lett. 9, 1476 共2009兲. 6 H. D. Lee, B. Magyrai-Köpe, and Y. Nishi, Phys. Rev. B 81, 193202 共2010兲. 7 J.-W. Park, J.-W. Park, D.-Y. Kim, and J.-K. Lee, J. Vac. Sci. Technol. A 23, 1309 共2005兲; R. Jung, M.-J. Lee, S. Seo, D. C. Kim, G.-S. Park, J.-S. Kim, and B. H. Park, Appl. Phys. Lett. 91, 022112 共2007兲. 8 I. Hotový, J. Huran, J. Janík, and A. P. Kobzev, Vacuum 51, 157 共1998兲. 9 S. Stanescu, C. Boeglin, A. Barbier, and J.-P. Deville, Phys. Rev. B 67, 035419 共2003兲. 10 D.-K. Kwon, K. K. Kim, J. H. Jang, J. M. Jeon, M. H. Lee, G. H. Kim, X.-S. Li, G.-S. Park, B. Lee, S. Han, M. Kim, and C. S. Hwang, Nat. Nanotechnol. 5, 148 共2010兲. 11 J. P. Strachan, M. D. Pickett, J. J. Yang, S. Aloni, A. L. D. Kilcoyne, G. Medeiros-Ribeiro, and R. S. Williams, Adv. Mater. 共Weinheim, Ger.兲 22, 3573 共2010兲. 12 The Condensed Chemical Dictionary, 10th ed., edited by B. G. G. Hawley 共Van Nostrand Reinhold, New York, 1981兲, p. 726. 13 P. R. Roberge, Handbook of Corrosion Engineering 共McGraw-Hill, New York, 2000兲, p. 1104. 1 (c) 0 0.0 grow to a certain extent wider than the comproportionation reaction can reach, the rupture of filaments can no longer be achieved. In summary, the RS of a polycrystalline NiO film involves the disproportionation/comproportionation reactions revealed by focused XPS analysis. Besides the formation/ rupture of Ni filaments with the SET/RESET process, Ni2O3 is produced/reduced as a consequence. The reversibility of the reactions can be thermodynamically explained by the Gibbs energy. From the CAFM result, the filaments expand/ contract with the forward/reverse reaction. The reaction becomes irreversible as the filaments grow larger than the range the reaction can reach. 0.8 FIG. 4. 共Color online兲 共a兲 I-V curves of RS of the NiO film with a scanning probe as the top electrode, 共b兲 CAFM mapping and its cross-section profile of an ON spot and an OFF spot, 共c兲 CAFM mapping and its cross-section profile of an irreversible ON spot. varies from 82 nm⫾ 3 nm for the ON spot to 53 nm⫾ 2 nm for the OFF spot. It indicates the spots contain multiple filaments and the filaments expand/contract with the SET/RESET process. Figure 4共c兲 shows the CAFM image and cross-sectional profile of an irreversible ON spot. The lateral dimension of the spot is about 240 nm 共three times larger than a normal spot兲 which is too large to be switched off. This shows that the dimension of the filaments increases very slightly in each RS cycle. When the filaments Downloaded 03 May 2011 to 129.234.252.65. Redistribution subject to AIP license or copyright; see http://apl.aip.org/about/rights_and_permissions