Enzymatic and non-enzymatic activation mechanisms of P AR
advertisement
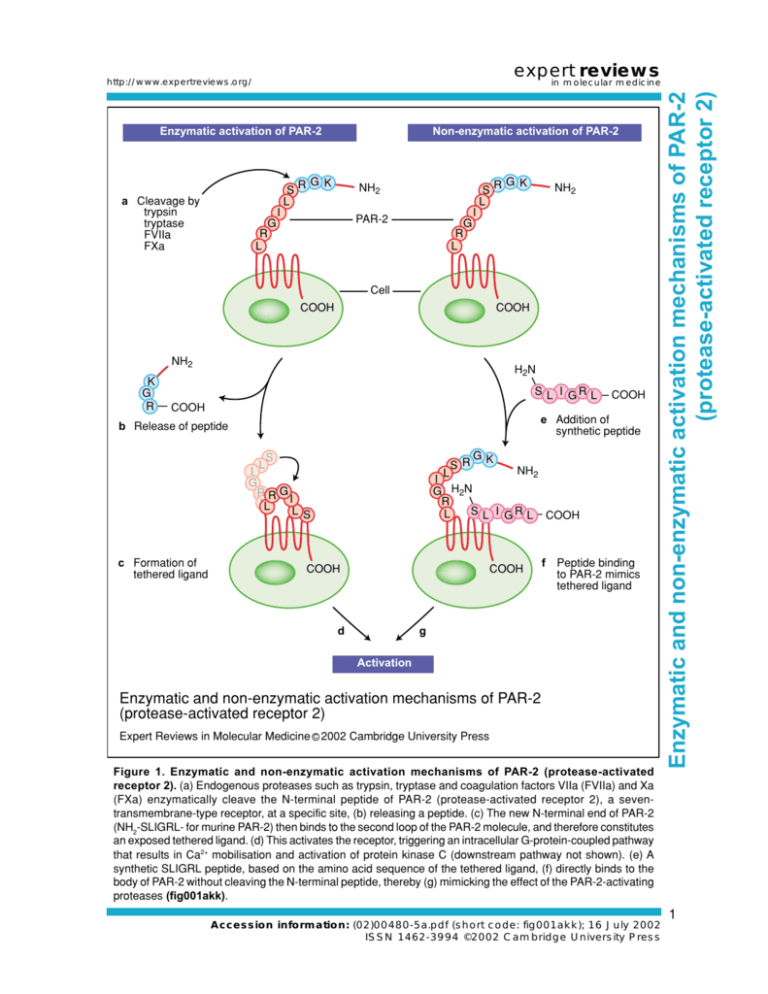
expert reviews http://www.expertreviews.org/ Enzymatic activation of PAR-2 S L a Cleavage by trypsin tryptase FVIIa FXa Non-enzymatic activation of PAR-2 RG K NH2 I S L NH2 I PAR-2 G R L RG K G R L Cell COOH COOH NH2 K G R H2N S L I GR L COOH COOH e Addition of synthetic peptide b Release of peptide S L I G RRG I LL L c Formation of tethered ligand SR GK NH2 L I G H2N R S L I G R L COOH L S COOH COOH d f Peptide binding to PAR-2 mimics tethered ligand g Activation Enzymatic and non-enzymatic activation mechanisms of PAR-2 (protease-activated receptor 2) Expert Reviews in Molecular Medicine C 2002 Cambridge University Press Figure 1. Enzymatic and non-enzymatic activation mechanisms of PAR-2 (protease-activated receptor 2). (a) Endogenous proteases such as trypsin, tryptase and coagulation factors VIIa (FVIIa) and Xa (FXa) enzymatically cleave the N-terminal peptide of PAR-2 (protease-activated receptor 2), a seventransmembrane-type receptor, at a specific site, (b) releasing a peptide. (c) The new N-terminal end of PAR-2 (NH2-SLIGRL- for murine PAR-2) then binds to the second loop of the PAR-2 molecule, and therefore constitutes an exposed tethered ligand. (d) This activates the receptor, triggering an intracellular G-protein-coupled pathway that results in Ca2+ mobilisation and activation of protein kinase C (downstream pathway not shown). (e) A synthetic SLIGRL peptide, based on the amino acid sequence of the tethered ligand, (f) directly binds to the body of PAR-2 without cleaving the N-terminal peptide, thereby (g) mimicking the effect of the PAR-2-activating proteases (fig001akk). Accession information: (02)00480-5a.pdf (short code: fig001akk); 16 July 2002 ISSN 1462-3994 ©2002 Cambridge University Press Enzymatic and non-enzymatic activation mechanisms of PAR-2 (protease-activated receptor 2) in molecular medicine 1








