OPDP Warning Letters: Pharma Advertising Violations (2011)
advertisement
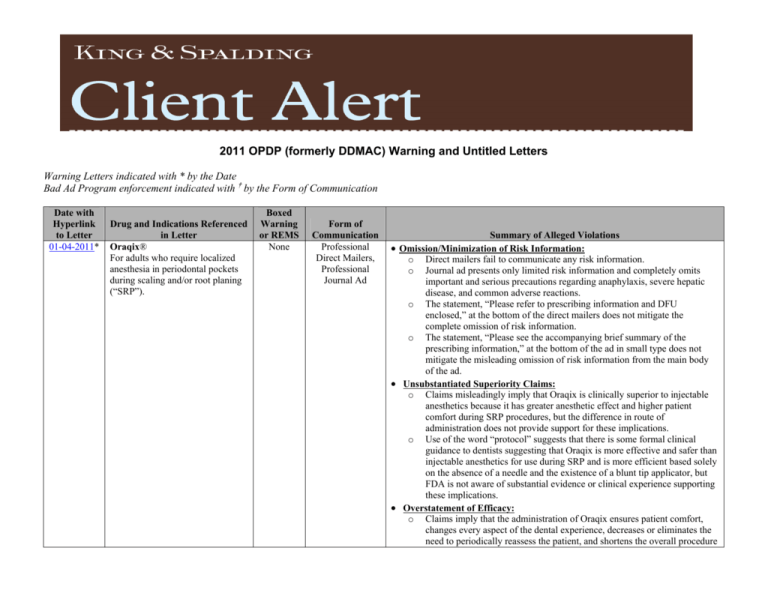
2011 OPDP (formerly DDMAC) Warning and Untitled Letters Warning Letters indicated with * by the Date Bad Ad Program enforcement indicated with † by the Form of Communication Date with Hyperlink to Letter 01-04-2011* Drug and Indications Referenced in Letter Oraqix® For adults who require localized anesthesia in periodontal pockets during scaling and/or root planing (“SRP”). Boxed Warning or REMS None Form of Communication Professional Direct Mailers, Professional Journal Ad Summary of Alleged Violations Omission/Minimization of Risk Information: Direct mailers fail to communicate any risk information. Journal ad presents only limited risk information and completely omits important and serious precautions regarding anaphylaxis, severe hepatic disease, and common adverse reactions. o The statement, “Please refer to prescribing information and DFU enclosed,” at the bottom of the direct mailers does not mitigate the complete omission of risk information. o The statement, “Please see the accompanying brief summary of the prescribing information,” at the bottom of the ad in small type does not mitigate the misleading omission of risk information from the main body of the ad. Unsubstantiated Superiority Claims: o Claims misleadingly imply that Oraqix is clinically superior to injectable anesthetics because it has greater anesthetic effect and higher patient comfort during SRP procedures, but the difference in route of administration does not provide support for these implications. o Use of the word “protocol” suggests that there is some formal clinical guidance to dentists suggesting that Oraqix is more effective and safer than injectable anesthetics for use during SRP and is more efficient based solely on the absence of a needle and the existence of a blunt tip applicator, but FDA is not aware of substantial evidence or clinical experience supporting these implications. Overstatement of Efficacy: o Claims imply that the administration of Oraqix ensures patient comfort, changes every aspect of the dental experience, decreases or eliminates the need to periodically reassess the patient, and shortens the overall procedure o o 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 01-07-2011 Drug and Indications Referenced in Letter Boniva® For the treatment and prevention of osteoporosis in postmenopausal women. Boxed Warning or REMS None Form of Communication Summary of Alleged Violations time, but FDA is not aware of substantial evidence or clinical experience to support these claims. Broadening of Indication: o Direct mailer suggests that Oraqix is safe and effective for use in any patient undergoing routine dental cleaning when this is not the case. o Direct mailer suggests that Oraqix is safe and effective for use in all patients undergoing SRP procedures, which is a broader range of conditions and patients than has been demonstrated by substantial evidence or clinical experience. o Misleading presentations are exacerbated by the failure to provide the complete limitations to the approved indication. Overstatement of Efficacy: Direct-toConsumer (“DTC”) Print Ad o o 02-17-2011 Feraheme® For the treatment of iron deficiency anemia in adult patients with chronic kidney disease. None Overstatement of Efficacy: Professional Direct Mailer o o o 03-11-2011 Ovide® For patients infected with Pediculus humanus capitis (head lice and their ova) of the scalp hair. None Claim suggests that treatment with Boniva will result in 9 out of 10 women stopping or reversing their bone loss when this is not supported by substantial evidence or clinical experience. Claim was based on a per-protocol post-hoc analysis of a secondary efficacy endpoint that the clinical study, which served as the basis for approval, was not adequately designed to evaluate. Claims suggest that increases in hemoglobin levels have been demonstrated specifically in patients without concomitant erythropoiesisstimulating agent (“ESA”) use when this finding has not been demonstrated by substantial evidence or clinical experience. None of the three pivotal trials used to evaluate the clinical efficacy of Feraheme were designed to evaluate efficacy based on ESA use and patients were not stratified by ESA use at randomization, so the conclusions based on sub-group analysis are considered exploratory and are not substantial evidence. The misleading impression is exacerbated by the complete omission of the results of the actual primary analyses from the studies. Omission of Risk Information: Book o 2 Book entirely omits all risk information associated with the product and misleadingly suggests that Ovide is safer than has been demonstrated. 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 03-21-2011* Drug and Indications Referenced in Letter Infergen® For treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease. Boxed Warning or REMS Boxed Warning Form of Communication STATgram† 3 Summary of Alleged Violations Omission/Minimization of Risk Information: o STATgram does not present all Boxed Warnings, contraindications to use of Infergen and ribavirin, most warnings and precautions, or common adverse events. o STATgram minimizes Boxed Warnings regarding neuropsychiatric, autoimmune, ischemic, and infectious disorders by failing to reveal that the reactions can be fatal or life-threatening and that patients should be monitored closely and withdrawn from therapy if they have persistently severe or worsening symptoms of the adverse events. o STATgram omits specific information from the Warnings and Precautions section of the Prescribing Information (“PI”) related to the risk of severe psychiatric adverse events. o Referring readers to the product website for safety information is insufficient to balance the efficacy claims in the STATgram. Broadening of Indication/Omission of Material Facts: o Claim implies that Infergen plus ribavirin is approved for retreating all patients infected with hepatitis C virus when this is not the case. o STATgram omits the material fact that the safety and efficacy of Infergen in combination with ribavirin have not been evaluated in treatment-naive patients or patients who are co-infected with hepatitis B virus or human immunodeficiency virus type 1. o STATgram omits material information that patients are less likely to benefit from retreatment with combination therapy if they have certain specific characteristics. o STATgram omits the fact that no safety and efficacy data are available for treatment with Infergen longer than one year. o Omission of material facts about limitations in Infergen’s indication is even more problematic given the serious safety risks associated with the product and the serious deficiencies in the safety information presented. Overstatement of Efficacy: o Claims regarding patient response are based on open-label studies that were not adequately powered to draw valid statistical conclusions about the efficacy of Infergen/ribavarin in particular subgroups in the overall study population. Unsubstantiated Claim: o Claim that patients maintaining full doses of Infergen/ribavarin therapy had a sustained virologic response of 17% is not supported by substantial evidence or clinical experience. 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 04-14-2011 04-26-2011 Drug and Indications Referenced in Letter Boxed Warning or REMS AzaSite® For the treatment of bacterial conjunctivitis caused by susceptible isolates of CDC coryneform group G, Haemophilus influenzae, Staphylococcus aureus, Streptococcus mitis group, and Streptococcus pneumoniae. None Nitrolingual® For acute relief of an attack or prophylaxis of angina pectoris due to coronary artery disease. None Form of Communication Summary of Alleged Violations Failure to Provide Adequate Directions for Use: o STATgram does not appear to have been disseminated with the full FDAapproved product labeling for Infergen. Broadening of Indication: Professional Journal Ad Totality of the presentation suggests that AzaSite is indicated to treat any condition that causes ocular surface damage, which has not been demonstrated by substantial evidence or clinical experience. o Presentation of the indication in small font on the bottom of the third page does not mitigate the overwhelming impression that AzaSite is useful in a much broader range of conditions or patients than has been demonstrated. Unsubstantiated Claims: o Claim implies that AzaSite delivers anti-inflammatory effects, which has not been demonstrated because the cited poster presentation does not provide adequate descriptions of the study materials, methods, or results, and the studies describe in vitro work on human cells and pre-clinical animal work, the clinical relevance of which is not known. o Other trials evaluated treatment efficacy based on clinical resolution and bacterial eradication and do not constitute substantial evidence to support claims regarding anti-inflammatory effects. o Claim implies that AzaSite has been shown to maintain therapeutic concentrations in ocular surface tissues for at least five days after the last dose, which has not been demonstrated because the cited poster presentation does not provide adequate descriptions of the study material, methods, or results. The study assessed pharmacokinetic parameters only and did not assess clinical efficacy, and the maintenance of therapeutic concentrations in ocular surface tissues was not a pre-specified endpoint in the trials. Omission/Minimization of Risk Information: o Ad omits warnings and precautions regarding the risk of anaphylaxis and hypersensitivity with systemic use of azithromycin. o Ad fails to present risk information with prominence and readability reasonably comparable to the presentation of efficacy claims. o Unsubstantiated Superiority Claims/Unsubstantiated Claims: Professional Sales Aid, Patient Brochure, Patient Brochure Holder o 4 Claims and presentations imply that Nitrolingual Pumpspray is more potent and provides faster pain relief from acute angina pectoris with fewer or no headaches compared to the nitroglycerin tablet formulation and is therefore clinically superior when this has not been demonstrated. 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication Summary of Alleged Violations A study cited in support of the claims is not substantial evidence or clinical experience because it used subjective questionnaires to assess the efficacy and safety of nitroglycerin lingual spray and nitroglycerin tablets. o Another study cited, an open-label trial of 20 healthy volunteers that compared the vasodilatory effects of the spray and tablet, does not constitute substantial evidence or clinical experience because it was not adequately powered, used an open-label design, and evaluated an endpoint that has unknown clinical relevance. o Claims that patients prescribed nitroglycerin tablets frequently carry subpotent tablets is not supported by substantial evidence or clinical experience because the cited patient survey did not provide information confirming that nitroglycerin tablets were in fact post-expiration or subpotent, as reported by patients. o Totality of claims implies that Nitrolingual Pumpspray is “patient-friendly” and that overall treatment is “convenient” when compared to tablets when this is not the case. Cited studies did not specifically assess patientfriendliness and overall convenience, both of which are broad terms that include many factors, and the PI describes multiple considerations for use and detailed instructions on administration. o Claim that Nitrolingual Pumpspray is not affected by dry mouth or diminished salivary secretions is not supported. o Claim implying that patients using Nitrolingual Pumpspray will not be “slow[ed] down” at all is not supported. Omission/Minimization of Risk Information: o Direction to use Nitrolingual Pumpspray “with caution” in patients who show hypersensitivity to it minimizes the contraindication in patient who are allergic to it. o Claim that Nitrolingual Pumpspray should be used with caution if patients have low systemic blood pressure minimizes the risk of severe hypotension in patients with low systolic blood pressure. o Sales aid omits the warning regarding use after an acute myocardial infarction and precautions regarding hypertrophic cardiomyopathy and tolerance associated with use of Nitrolingual Pumpspray. o Brochure and brochure holder completely omit important information such as a precaution regarding tolerance associated with use of Nitrolingual Pumpspray. o Brochure and brochure holder fail to present risk information with a prominence and readability comparable with the presentation of efficacy information. o 5 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 04-28-2011 04-28-2011 Drug and Indications Referenced in Letter Savella® For the management of fibromyalgia. Omapro™ (Investigational New Drug) Boxed Warning or REMS Boxed Warning None Form of Communication Oral Statements by Sales Representative† Summary of Alleged Violations Promotion of Unapproved Uses: o Sales representative made an unsolicited sales call to a physician office and stated that Savella is useful in back pain and mood disorder and approved for depression in Europe, but FDA is not aware of substantial evidence or clinical experience supporting use for back pain, mood disorder, or depression. Unsubstantiated Superiority Claims/Minimization of Risk: o Sales representative stated that Savella has a 3:1 affinity for norepinephrine reuptake inhibition, which makes it a better analgesic than Cymbalta, but pre-clinical data demonstrating Savella’s increased affinity for norepinephrine reuptake inhibition does not constitute substantial evidence of superiority. o Sales representative stated that Savella is less sedating and does not result in peripheral edema and/or as much weight gain compared to Lyrica, but FDA is not aware of any adequate and well-controlled head-to-head trials comparing these effects of Savella versus Lyrica. o Statements comparing the safety of Savella versus Lyrica and failure to mention any of the most serious and common risks associated with use of Savella suggest that the drug is safer than has been demonstrated. Unsubstantiated Mechanism of Action Claim: o Statement that the increase in spinal cord norepinephrine provides analgesia for Savella and is the mechanism of action that should be targeted as the goal for use of centrally acting analgesics implies a greater deal of certainty about the mechanism of action of Savella than is supported by substantial evidence or clinical experience. The exact mechanism of the central pain inhibitory action of Savella and its ability to improve symptoms of fibromyalgia in humans is unknown. Promotion of an Investigational New Drug: Brochure Claims promote Omapro for treatment of chronic myelogenous leukemia and other hematologic malignancies, but Omapro has not yet been demonstrated to be safe and effective for treatment of these conditions in patients. False or Misleading Statements: o Statements touting positive clinical trial data are false or misleading. o FDA is not aware of the establishment of expanded compassionate use access for Omapro, as claimed in the brochure. o 6 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 05-05-2011 05-06-2011* Drug and Indications Referenced in Letter Atelvia™ For the treatment of osteoporosis in postmenopausal women. Vyvanse® For the treatment of Attention Deficit Hyperactivity Disorder (“ADHD”) in children. Boxed Warning or REMS None Boxed Warning Form of Communication YouTube Video† Summary of Alleged Violations Omission of Indication and Risk Information: o Video fails to communicate any of the risks associated with use of Atelvia, including contraindications, warnings and precautions, and adverse reactions. o Video fails to communicate Atelvia’s indication. Omission of Material Facts/Misleading Claims Regarding Dosing: o Video omits material facts about dosing. o Video implies that patients have a choice to eat and drink when taking Atelvia when the Dosage and Administration section of the PI states that Atelvia should be taken immediately following breakfast. Failure to Submit Under Form FDA-2253: o Video was not submitted to FDA at the time of initial dissemination or publication. Omission of Indication and Risk Information: Magnet† o o o 05-24-2011 Pexeva® For the treatment of Major Depressive Disorder (“MDD”), obsessions and compulsions in patients with Obsessive Compulsive Disorder (“OCD”) as defined in the DSM-IV, Panic Disorder (“PD”), with or without agoraphobia, as defined in DSMIV, and Generalized Anxiety Disorder (“GAD”), as defined in DSM-IV. Boxed Warning Magnet fails to adequately communicate the full indication for Vyvanse, including important information on special diagnostic considerations, the need for comprehensive treatment, and long-term use. Magnet fails to adequately disclose risk information associated with the use of Vyvanse because a majority of the risk information is covered by a sales representative business card. The statement, “Please see full accompanying Prescribing Information, including Boxed Warning,” at the bottom of the magnet does not mitigate the omission of indication and risk information. Broadening of Indication/Unsubstantiated Claims: Flashcard o o 7 Flashcard presentation implies that Pexeva is effective in treating patients with co-morbid MDD and GAD when no clinical studies demonstrate efficacy of Pexeva in treating patients experiencing the two conditions concurrently, and co-morbid MDD and GAD is not recognized as a distinct clinical entity in the DSM-IV-TR or in the academic or clinical community. Study cited to support a claim that anxiety symptoms are associated with MDD is not substantial evidence or clinical experience because the study was a cross-sectional study that only evaluated efficacy endpoints related to depression scales. No scales for GAD were included, nor were any corrections for multiple statistical comparisons performed, and the purpose of the study was to directly compare the clinical features of depression for patients entering clinical trials at primary and specialty care settings, not to 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication Summary of Alleged Violations identify anxiety disorders distinct from MDD. o Inclusion of the statement, “Pexeva is indicated for the treatment of MDD, GAD, Panic Disorder, and OCD,” in the bottom corner of the flashcard does not mitigate the misleading impression. Overstatement of Efficacy: o Flashcard presentation implies that Pexeva is efficacious in treating certain individual symptoms, but FDA is not aware of any substantial evidence or clinical experience to support the claim that patients will experience improvement in individual symptoms because clinical trials showed that Pexeva was effective based on the total scores of scales evaluating mood disorder. o The statement, “These selected symptoms of GAD, MDD, OCD, and PD are used here only as examples,” does not mitigate the misleading presentation. Omission/Minimization of Risk Information: o Flashcard entirely omits the warning regarding potentially fatal serotonin syndrome and neroleptic malignant syndrome-like reactions, as well as precautions related to the activation of certain adverse events upon discontinuation of treatment with Pexeva, akathisia, hyponatremia, and abnormal bleeding. o Flashcard fails to include important material facts from the Warnings section that the contraindication in patients taking monoamine oxidase inhibitors and thioridazine is due to serious, potentially fatal interactions that may result from concomitant use of Perexa and the drug products. o Disclosures about suicidality and antidepressant drugs in the Boxed Warning fail to include the material fact that families and caregivers should be advised of the need for close observation and communication with the prescriber, and that Pexeva is not approved for pediatric patients. o Flashcard fails to include important information from the Warnings section that all patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy or at times of dose changes. o The statement, “Please see accompanying full Prescribing Information including WARNING – Clinical Worsening and Suicide Risk,” does not mitigate the misleading impression. o Claim of “[s]ignificant improvements in associated sleep disturbances” is misleading because it fails to include the material fact that Perexa is associated with a 13% incidence of insomnia in MDD patients. 8 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication o 05-31-2011 Focalin XR® For the treatment of ADHD in patients aged 6 years and older. Boxed Warning Unsubstantiated Superiority Claims: Professional One Point Leave Behind Detail Aid o o 06-16-2011 Acanya® For the topical treatment of acne vulgaris in patients 12 years and older. None Summary of Alleged Violations Presentation of insomnia as an adverse event somewhere else on the flashcard is too far removed from the claims and presented at the bottom of the page with other risk information. Claims imply that Focalin XR is superior to Concerta because of a benefit demonstrated at 2 hours post-dose. However, the referenced clinical studies do not constitute substantial evidence or clinical experience to support the claims because the studies only focused on one specific time point as the primary efficacy measure and did not account for the different pharmacokinetic profiles and subsequent efficacy profiles associated with Focalin XR and Concerta over the entire treatment course. Detail aid implies that Focalin XR is better or more effective than other ADHD medications and should be the “first” choice when considering treatment options, but this claim is not supported by substantial evidence or clinical experience. Overstatement of Efficacy: Website Claims and presentations imply a substantial effect of Acanya at 2 weeks and continued improvement throughout a 12-week treatment period, but the clinical study cited does not constitute substantial evidence or clinical experience to support the claim because the study had co-primary efficacy variables measured at week 12. Patients were evaluated at weeks 4 and 8, but the earlier timepoints were not pre-specified endpoints in the clinical studies. o The “Acanya Gel: Consumer Home Page” and subsequent pages include large images of faces depicting completely clear, acne-free skin that conveys the impression that treatment with Acanya will result in complete clearing of acne when this has not been demonstrated by substantial evidence or clinical experience. o Presentation of before and after photos followed by the statements, “These unretouched photos represent actual clinical trial experience. As with all treatments, results may vary from person to person,” does not mitigate the misleading impression. Omission of Material Facts/Minimization of Risk Information: o Website communicates that patients with a history of colitis should not use Acanya, but fails to communicate that use of the drug is contraindicated in patients with a history of Crohn’s disease. o Website indicates that the drug “may cause diarrhea” and that patients who o 9 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication o o o o o 06-17-2011 Solaraze® For the topical treatment of actinic keratoses (“AKs”). None Summary of Alleged Violations experience diarrhea should stop using Acanya immediately and call their doctor, but fails to convey that severe abdominal cramps are another symptom of colitis. Website fails to convey that Acanya may cause serious allergic reactions and that patients who experience allergic reactions should stop using Acanya and call their doctor right away. Website does not present risks in order of severity. Inclusion of a link to the full PI does not mitigate the misleading impression. Claim implies that Acanya is gentle to the skin when clinical trials showed up to 8% and 2% of patients experienced mild and moderate burning, respectively, up to 6% and 1% experienced mild and moderate stinging, respectively, and mild and moderate erythema, scaling, and itching were common local side effects. The statement, “Side effects may include redness, scaling, itching, burning, and stinging,” presented at the bottom of the webpage does not mitigate the misleading impression of the claim. Overstatement of Efficacy: Flashcard Flashcard overstates the efficacy of Solaraze in clearing target lesions and is inconsistent with the efficacy results demonstrated in the Clinical Studies section of the PI. o Claims regarding the efficacy of Solaraze 1 year post-treatment are not supported by the approved PI, which specifically states that “No long-term patient follow-ups, after the 30-day assessments, were performed for the detection of recurrence,” and FDA is not aware of substantial evidence to support claims that Solaraze has a long-lasting treatment effect after use has been discontinued. o Claims that Solaraze is effective in treating subclinical lesions are misleading because the efficacy of Solaraze in treating subclinical lesions was not studied. o Claim that Solaraze has been demonstrated to prevent recurrence of AKs is not supported by substantial evidence or clinical experience and is inconsistent with the PI, which states that no long-term follow-ups were performed after 30-day assessments. Minimization of Risk: o Claim that Solaraze is “well-tolerated” minimizes the adverse events associated with Solaraze use and is not supported by the PI, which describes adverse events involving skin and the application site in 83% of o 10 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 06-21-2011 Drug and Indications Referenced in Letter Trisenox® For induction of remission and consolidation in patients with acute promyelocytic leukemia (“APL”) who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha [promyelocytic leukemia/retinoic acid receptor-alpha] gene expression. Boxed Warning or REMS Form of Communication Boxed Warning Professional Website Summary of Alleged Violations treated patients and discontinued participation in clinical trials mainly due to skin irritation of cutaneous adverse reactions. Misleading Patient Compliance Claim: o Claim that a “[m]ajority of patients were compliant with treatment” is not supported by the cited reference, which was an open-label study designed to assess the efficacy of Solaraze for treatment of AKs and not patient compliance with Solaraze. Broadening of Indication: Prominent, bolded headers at the top of the webpages suggest that Trisenox is approved to treat patients with any kind of relapsed or refractory APL when this is not the case. o Inclusion of the full indication statement in small type on the bottom of each webpage beneath the list of references does not mitigate the misleading impression created by the bolded headers. Minimization of Risk: o Information about serious and potentially fatal risks associated with Trisenox, including the Boxed Warning, are relegated to the bottom of the pages after the list of references and written in small single-spaced font, which is not with a prominence or readability reasonably comparable to effectiveness claims. o Website understates several risk-related statements by listing below the headline, “Safety Profile for Trisenox,” claims that emphasize the absence or low incidence of selected side effects instead of the most serious and common risks. o Information from the Boxed Warning and that fact that serious adverse events were common are relegated to the bottom of the page after a list of references. o Characterization of the serious and significant risks associated with Trisenox as “manageable” and “generally well tolerated” minimizes the risks and contributes to the overall suggestion that Triselox is safer than has been demonstrated. o Statement misleadingly suggests that Trisenox is safer than chemotherapy by implying that Trisenox does not expose patients to cytotoxic adverse effects and is not associated with potentially fatal side effects when the Boxed Warning and Warning and Precautions section of the PI describes severe side effects, some of which may be fatal, and states that serious adverse reactions were common in patients taking Trisenox. o Suggestions that Trisenox should be considered before chemotherapy for o 11 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication 12 Summary of Alleged Violations young patients on the basis of the implied lower toxicity is unsubstantiated, and the PI explicitly states that there is “limited data about the pediatric use of Trisenox” and that “the safety and efficacy of Trisenox has not been studied in patients younger than four years of age.” Overstatement of Efficacy: o Website misleadingly overstates the efficacy of Trisenox because it is based on a retrospective recalculation of the original efficacy results using new, less stringent criteria that make the response rates appear more favorable than those reported in the PI. o While calculation criteria may have been revised since the time of the pivotal study, approval of Trisonex was based on earlier-established criteria. It is misleading to promote inflated response rates that are inconsistent with the efficacy findings from pivotal clinical studies for the drug, as reflected in the PI. o Claims related to overall survival and relapse-free survival are not supported by substantial evidence or clinical experience because pivotal studies did not measure either as endpoints and time-to-event analyses are not interpretable in a single-arm clinical trial. Furthermore, median follow-up times was 16, not 18, months as claimed. o Statements reference clinical studies performed in patients with APL who were treated with all-trans retinoic acid or various chemotherapy agents during induction, consolidation, or salvage therapy, and suggest that patients treated with Trisenox will experience similar survival outcomes based on their molecular response status, but the correlation between levels of PML/RAR-alpha transcript and the probability of relapse or relapse-free survival were not pre-defined endpoints in the Trisenox pivotal trial. Unsubstantiated Claims: o Several claims in an animated video relate to the mechanism of action of Trisenox, but according to the PI, the mechanism of action is not completely understood; the PI does not indicate that Trisenox selectively targets and degrades the PML/RAR-alpha protein in vivo or that these functions confer a clinical benefit to patients with APL. o Cited references describe in vitro studies performed in cell culture with different concentrations of arsenic trioxide, but these finds do not necessarily correlate to in vivo activity or support any claim of clinical benefit. o Inclusion in the animated video of the statement, “[t]he mechanisms of action of Trisenox are not completely understood and are actively studied,” is insufficient to mitigate the misleading implications in the video. 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter Drug and Indications Referenced in Letter Boxed Warning or REMS Form of Communication Summary of Alleged Violations Misleading Claims: Claims that dosing or administration of Trisenox is “manageable” are misleading given the fact that patients may be spending four hours a day, every day, for two months being infused with the medication. o Given the extent to which patients must be monitored and the potential for serious adverse effects if they are not carefully monitored, it is misleading to characterize the complex regimen as “manageable” for patients and their providers. Omission of Risk: o Website fails to include important risk concepts from the Warnings and Precautions sections of the PI, including warnings that Trisenox is a human carcinogen and may cause fetal harm when administered to pregnant women and the precaution against using Trisenox when nursing. o 06-30-2011 KRX-0401 (Perifosine) (Investigational New Drug) None Promotion of an Investigational Drug: Website o o o 07-13-2011* Bromday™ For the treatment of postoperative inflammation and reduction of ocular pain in patients who have undergone cataract surgery. None Website makes numerous statements that promote KRX-0401 as safe and/or effective for treatment of various types of tumors, both as a single agent and in combination with other therapies when it has not been approved for these uses. The totality of claims makes the conclusions that the drug is well-tolerated, can be safely given to humans with a manageable toxicity profile, and has a safety profile that is “distinctly different from that of most cytotoxic agents” when the safety and effectiveness of KRX-0401 have not been established yet. A disclaimer stating, “This investigational drug product has not been approved by the US Food and Drug Administration for safety and effectiveness. This investigational drug product is still undergoing clinical study to verify its safety and effectiveness” is not sufficient to mitigate the misleading impression conveyed by claims on the website. Omission of Risk Information: Flyer o o o 13 Flyer discloses the most common adverse reactions associated with use of Bromday, but fails to reveal any of the warnings and precautions for the drug, including serious and important warnings and precautions regarding the potential for anaphylactic and life-threatening allergic reactions to sodium sulfite and other adverse reactions. Flyer fails to communicate the warning and precaution that Bromday should not be administered while wearing contact lenses. Inclusion of the statement, “Please see full prescribing information on 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 08-02-2011 08-05-2011* Drug and Indications Referenced in Letter Boxed Warning or REMS Mephyton® For the treatment of certain coagulation disorders that are due to faulty formation of factors II, VII, IX, and X when caused by Vitamin K deficiency or interference with Vitamin K activity. None Multikine® (Investigational New Drug) None Form of Communication Booklet Containing Slide Presentation Summary of Alleged Violations reverse,” at the bottom of the flyer does not mitigate the omission of risk information. Omission of Material Facts: o Flyer fails to reveal important dosing limitations that are material to the safe use of the drug. o The omission is particularly concerning in light of the claim that Bromday is the replacement for Xibrom, which suggests that Bromday has the same dosing regimen that was previously approved for Xibrom when this is not the case. o Omission of dosing information is concerning because the Warnings and Precautions section of the PI states that use of topical non-steroidal antiinflammatory drugs more than 24 hours prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events. Broadening of Indication: Booklet presentation creates the impression that Mephyton is indicated for all patients with Vitamin K deficiency when this has not been demonstrated. o Presentation of Mephyton’s indication on other slides does not mitigate the misleading impression of the presentation. Unsubstantiated Claims: o Booklet suggests that at 24 hours post-administration, Mephyton is as clinically effective as intravenously administered phytonadione when this has not been demonstrated by substantial evidence or clinical experience because the cited study is a retrospective review of multiple clinical studies that were performed in diverse patient populations with different doses and dosage forms of phytonadione under varying clinical protocols. o Promotion of Investigational New Drug: Webpages o o 14 Claims suggest that Multikine is safe and/or effective for the treatment of various kinds of cancers, including those of the head and neck, when it has not been approved for such uses. Totality of claims suggest that the drug is “non-toxic” and has demonstrated “impressive” and “extraordinary” improvements in overall survival “over what can be expected from the current therapy,” but the indications, warning, precautions, adverse reactions, dosage, and administration have not been established yet and are currently unknown. 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 08-26-2011 Drug and Indications Referenced in Letter Nucynta® For the relief of moderate to severe acute pain in patients 18 years of age or older. Boxed Warning or REMS None Form of Communication Oral Statements Made by Representative 15 Summary of Alleged Violations Promotion of Unapproved Uses: o Sales representative indicated that Nucynta is useful in the treatment of Diabetic Peripheral Neuropathic Pain (“DPNP”) when Nucynta is only indicated generally for relief of moderate to severe pain in patients 18 years of age or older and not specifically for the treatment of DPNP, a chronic pain condition. Unsubstantiated Superiority Claims/Minimization of Risk: o Sales representative indicated that Nucynta is clinically superior (i.e., safer) compared to oxycodone and tramadol for DPNP patients, and that Nucynta has been shown to have fewer gastrointestinal adverse reactions in comparison to oxycodone and/or tramadol when FDA determined that clinical studies for Nucynta were not adequately powered for analysis of multiple safety endpoints and that doses of oxycodone used as a comparator were not demonstrated to be equianalgesic to the doses of Nucynta studied, so safety comparative data were not considered clinically meaningful or included in the approved PI for Nucynta. o FDA is not aware of any adequate and well-controlled head-to-head clinical trials comparing the incidence of constipation, nausea, or vomiting for Nucynta versus tramadol. o Claim that Nucynta results in less constipation, nausea, and vomiting minimizes the risks associated with use because nausea and vomiting were the most common adverse reactions associated with use of Nucynta in clinical trials and among the most common reasons for discontinuation of treatment. o Sales representative implied that treatment with Nucynta has been shown to reduce length of hospital stay in comparison to oxycodone and tramadol and that Nucynta-treated patients will have a bowel movement without use of docusate or senna, but 8% of Nucynta-treated patients reported constipation as an adverse event in clinical studies versus 3% in the placebo arm. The lack of support comparing the safety and efficacy of Nucynta to oxycodone and tramadol makes it impossible to make a treatment cost comparison based on the length of hospital stay. Unsubstantiated Efficacy Claim: o Sales representative implied that Nucynta has been shown to be noninferior to oxycodone, tramadol, or other opiods and that Nucynta has been shown to provide equivalent “pain control” (equianalgesia) when compared to other opiods, but FDA determined that analyses used to obtain non-inferiority claims regarding the efficacy of Nucynta compared to oxycodone were inadequate, so the results of those analyses were excluded 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 08-31-2011 Drug and Indications Referenced in Letter Chantix® For use as an aid to smoking cessation treatment. Boxed Warning or REMS Both (Chantix) Form of Communication Summary of Alleged Violations from the approved PI. FDA is not aware of any well-controlled head-tohead clinical trials comparing the efficacy of Nucynta to tramadol or any other opioids. Omission of Risk Information: Webpage† o o Caduet® For the treatment of patients for whom treatment with both amlodipine and atorvastatin is appropriate. “Online Resources” webpage for Lipitor fails to communicate any risk information about the products. Inclusion of links that lead to the Lipitor site, which contains a link to the individual product websites for Caduet and Chantix and the PI for Norvasc is insufficient to mitigate the omission of risk information in the “Online Resources” webpage. Norvasc® For the treatment of hypertension and vasospastic angina [Prinzmetal or variant angina] and the symptomatic treatment of chronic stable angina. 10-14-2011 Pataday™ For the treatment of ocular itching associated with allergic conjunctivitis. None Inappropriate Reminder Labeling/Omission of Indication and Risk Rebate Card Information: o Rebate card makes a representation regarding the use of Pataday for the “relief” of symptoms of allergic conjunctivitis caused by plant allergens and presentation of the Pataday logo in conjunction with the words “Once Daily” makes a dosage recommendation for the drug product, so the card is not considered reminder labeling and needs to include appropriate risk information and full indication information. o Rebate card fails to include the fact that Pataday is only indicated for the treatment of ocular itching associated with allergic conjunctivitis. o Inclusion of the statement, “For prescribing information visit pataday.com,” is not sufficient to mitigate the rebate card’s omission of risk and indication information. Unsubstantiated Superiority Claim: o Image of the eye with a superimposed plant allergen accompanied by the claim of “Most Relief” suggests that Pataday provides superior relief as compared to other available therapies approved for the same indication, but OPDP is not aware of substantial evidence or clinical experience 16 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 10-17-2011 Drug and Indications Referenced in Letter Busulfex® For use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation for chronic myelogenous leukemia. Boxed Warning or REMS Boxed Warning Form of Communication Summary of Alleged Violations supporting the claim. Omission of Material Facts: Website Website omits risks associated with use of Busulfex in pediatric patient populations, such as the risk of cardiac tamponade in pediatric patients receiving high doses of oral busulfan and other adverse events. o The “Important Safety Information” section of the website omits material facts regarding hepatic veno-occlusive disease (“HVOD”) and hepatotoxicity. o The “Dosing and Straightforward Administration” webpage omits material facts regarding the need to pre-medicate patients with antiemetics prior to the first dose of Busulfex therapy and on a fixed schedule for the duration of Busulfex treatment. Minimization of Risk/Unsubstantiated Claims: o Website claims that Busulfex has a “[l]ow incidence of severe toxicities,” but the Boxed Warning indicates that Busulfex is a cytotoxic drug associated with profound myelosuppression that occurs in all patients and includes other significant warnings and precautions. o Website presentation suggests that the use of lorazepam for seizure prophylaxis eliminates the risk of seizures with Busulfex therapy when this is not demonstrated by substantial evidence or clinical experience because the referenced study is a retrospective analysis in a limited pediatric population. This suggestion is also contrary to a recommendation in the Dosage and Administration section of the PI that phenoytoin be used because busulfan is known to induce seizures, and a statement that use of other anticonvulsants may result in increased risk of HVOD or seizures. o Totality of presentation is concerning due to a warning in the Busulfex PI regarding the risk of seizures. Unsubstantiated Claims: o Claims and presentations imply that Busulfex has a “predictable pharmacokinetic profile,” “predictable and consistent” area under the curve (“AUC”) values, and “excellent interdose reproducibility” when the referenced study used a sampling schedule that caused invalid comparisons of AUC values and dose values that differed significantly and prevented accurate Cmax predictions. o Totality of the presentation misleadingly implies that there is a direct correlation between a “predictable pharmacokinetic profile” and the ability to induce myeloablation in an “accurate,” “controlled,” and “optimal” way when OPDP is not aware of any substantial evidence supporting such a o 17 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 10-25-2011 Drug and Indications Referenced in Letter TechneLite® For use in adults for brain imaging, thyroid imaging, salivary gland imaging, placenta localization, blood pool imaging, urinary bladder imaging, and nasolacrimal drainage system imaging, and for use in children as an agent for brain imaging, thyroid imaging, blood pool imaging, and urinary bladder imaging. Boxed Warning or REMS Form of Communication None Exhibit Panel Summary of Alleged Violations correlative relationship. Overstatement of Efficacy: o Kaplan-Meier graph titled, “Overall Survival and Disease-Free Survival,” makes efficacy claims regarding the probability of overall survival (“OS”) and disease-free survival (“DFS”) following Busulfex therapy, but the referenced publication calculates probability estimates for these endpoints in a way that does not accurately reflect the number of patients still at risk for the events, which results in an overestimate of the probability of OS and DFS. The graph is not supported by the Busulfex PI either because the single-arm open-label pivotal study does not adequately characterize timeto-event endpoints and did not calculate OS and DFS probability estimates. Misleading Claim: o Claim regarding straightforward IV administration is misleading because there are several instructions required for proper administration of Busulfex. o Presentation of administration information in the website is not sufficient to mitigate the misleading impression of the claim. Omission of Risk Information: Exhibit panel fails to include any of the warnings and precautions associated with use of the drug, though the “Important Safety Information” section of the panel includes a statement about allergic reactions. o The statement, “Please see a representative in this booth for full Prescribing Information,” does not mitigate the omission of information. Unsubstantiated Superiority Claim: o Exhibit panel suggests that TechneLite is favored over other available generators for the indicated uses, which has not been demonstrated by adequate and well-controlled head-to-head studies. Unsubstantiated Claims: o Statement regarding simple, hassle-free use is misleading because the TechneLite PI describes a complex series of steps that are required for proper preparation and administration of TechneLite and safe use and disposal of radioactive material. Inadequate Presentation of Established Name: o Exhibit panel fails to present the established name of the product (Technetium Tc 99m Generator) in conjunction with the proprietary name (TechneLite). o 18 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 12-13-2011 12-13-2011 12-14-2011 Boxed Warning or REMS None Form of Communication Fact Sheet Qutenza® For the management of neuropathic pain associated with postherpetic neuralgia. None Exhibit Booth Latuda® For the treatment of patients with schizophrenia. Boxed Warning Drug and Indications Referenced in Letter ProstaScint® For use as a diagnostic imaging agent in newly diagnosed patients with biopsy-proven prostate cancer thought to be clinically localized after standard diagnostic evaluation and who are at high-risk for pelvic lymph node metastases, and for use as a diagnostic imaging agent in post-prostatectomy patients with a rising prostatespecific antigen and a negative or equivocal standard metastatic evaluation in whom there is a high clinical suspicion of occult metastatic disease. Summary of Alleged Violations Omission of Risk Information: o Fast sheet omits all contraindications, important warnings and precautions, and the most commonly reported adverse reactions associated with the use of ProstaScint. Overstatement of Efficacy: o Claims suggest that ProstaScint is effective for confidently determining specific treatment options (i.e., definitive and salvage) or as a prognostic indicator for prostate cancer patients when this has not been demonstrated by substantial evidence or clinical experience; two phase three trials found overall accuracy of interpreted ProstaScint images to be 63% in patients with high clinical suspicion for occult recurrent or residual prostate cancer and 68% in patients with clinically localized prostate cancer who were at high risk for metastases. The PI warns that patient management should not be based on ProstaScint scan results without appropriate confirmatory studies due to the high rate of false positive and false negative image interpretations in pivotal trials, and that ProstaStint imaging should be considered in conjunction with other diagnostic information. Inadequate Communication of Indication: o Fact sheet fails to adequately communicate ProstaScint’s full approved indication. De Facto Omission of Risk Information: o Risk information included in the exhibit booth was presented at the bottom of the display panels and behind bags, boxes, and other materials, which completely obscured the information from view. Promotion of Unapproved Uses: Oral Statements Made by a Sales Representative Sales representative indicated in a sales call that studies of Latuda use for bipolar disorder are being done, that it is only a matter of time before Latuda is approved for bipolar disorder, and that two area psychiatrists use Latuda for bipolar disorder and are pleased with the results, but Latuda is only indicated for treatment of schizophrenia. o Statement that Latuda is only approved for schizophrenia and that use for treatment of bipolar disorder is off-label does not mitigate the misleading impression that Latuda is safe and effective for treatment of bipolar disorder. Minimization of Risk Information/Unsubstantiated Claim: o Sales representative minimized the risk of common adverse reactions and stated that somnolence can occur during the beginning of treatment, but o 19 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 12-22-2011 Drug and Indications Referenced in Letter Colcrys® For prophylaxis and treatment of acute gout flares. Boxed Warning or REMS None Form of Communication Summary of Alleged Violations usually goes away after a week when the PI does not include information indicating a decreased risk of somnolence over time, includes a warning and precaution regarding the potential for cognitive and motor impairment, and reports somnolence as one of the most common adverse reactions associated with use of Latuda. Omission and Minimization of Risk Information: Pharmacy Sell Sheet and Professional Video Sell sheet and video omit material facts regarding the risk of rhabdomyolysis. o Sell sheet presentation implies that Colcrys is not risky or harmful (i.e., “gentle”) when this is not supported by substantial evidence or clinical experience and the PI lists serious risks. o Sell sheet and video imply that the safety profiles of Colcrys and placebo are not different or that the difference is minimal when the adverse reactions section of the PI states that 23% of patients in the recommended low-dose group experienced diarrhea versus 14% of the placebo group. o The main part of the video omits a discussion of the serious and significant risks and the most commonly reported adverse reactions, and risk information is not presented with a prominence and readability reasonably comparable to the presentation of effectiveness information. Minimization of Risk Information/Unsubstantiated Safety Superiority Claims: o Video suggests that there are not significant safety concerns for Colcrys in patients who have comorbid conditions and who are taking concomitant medications, but this has not been demonstrated by substantial evidence or clinical experience. o Presentation misleadingly implies that Colcrys is clinically superior (i.e., safer) in patients with comorbid conditions and has fewer drug interactions when taken with concomitant medications compared to non-steroidal antiinflammatory drugs, but OPDP is not aware of any adequate and wellcontrolled head-to-head studies supporting these claims, and the PI includes warnings and precautions regarding fatal overdoses, blood dyscrasias, life-threatening and fatal drug interactions, and neuromuscular toxicity. Overstatement of Efficacy: o Video implies that Colcrys effectively reduces pain associated with gout flares within 16 hours when this has not been demonstrated by substantial evidence or clinical experience because the clinical trial in the PI assessed efficacy at 24 hours following the time of first dose, was not appropriately o 20 2222011 OPDP (formerly DDMAC) Warning and Untitled Letters Date with Hyperlink to Letter 12-22-2011 Drug and Indications Referenced in Letter Fondaparinux Sodium Solution For the prophylaxis of deep vein thrombosis that may lead to pulmonary embolism in patients undergoing hip fracture surgery, hip replacement surgery, knee replacement surgery, or abdominal surgery who are at risk for thromboembolic complications; treatment of acute deep vein thrombosis when administered in conjunction with warfarin sodium; or treatment of acute pulmonary embolism when administered in conjunction with warfarin sodium when initial therapy is administered in the hospital. Boxed Warning or REMS Form of Communication Boxed Warning Professional Website Summary of Alleged Violations powered to evaluate outcomes at the 16-hour time point, and did not apply appropriate pre-specified adjustments for multiple comparisons between treatment groups during analysis of the study data. Minimization of Risk Information: o o o 21 Website fails to prominently display the Boxed Warning for the drug, which is not presented until the bottom of the webpage. The website navigation tab appears to list all sections of the PI, but does not include the Boxed Warning section. Boxed Warning risks are conveyed as part of the patient video and text at the very bottom of the website, but the overall effect of the presentation undermines the communication of the Boxed Warning.