determination of osmotic concentration in potato
advertisement

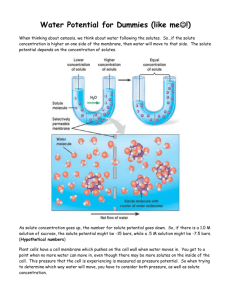
DETERMINATION OF OSMOTIC CONCENTRATION IN POTATO (Solanum tuberosum) PARENCHYMA By Dr. Elaine Winshell Updated and Revised by Dr. Susan Petro Objectives: • To understand that water moves across a cell membrane from a higher concentration to a lower one. • To understand what is meant by hypotonic, hypertonic and isotonic solutions. • To practice using Excel to calculate the mean and standard deviation. • To practice linear regression graphing and using the graph-derived equations to determine the osmotic concentration of the potato parenchyma (solving for x when you know y) Introduction: Living cells are bounded by selective membranes that allow certain substances in and exclude others. One substance that can easily traverse a membrane is water. Water will move from high water potential (pure water) to lower water potential (solute dissolved in it). Therefore when a tissue is placed in an environment in which the solute concentration is lower and the water concentration is greater than that within the tissue, there will be a net inward movement of water and the cells will swell. The solution is called hypotonic. When a tissue is placed in a solution in which the solute concentration is higher and the water concentration is lower than that in the cells, water will move out of the cells and the cells will shrink. Such an environment is called hypertonic. When the water concentrations and solute concentrations on the outside and inside are equal (isotonic), there will be no net movement of water. One way of determining the concentration of solute particles in the cell is to place the tissue in a variety of osmotic environments and determine which one causes no net uptake of water. Procedure • Using a #5 cork borer, cut potato tuber cylinders from the potato. With a razor blade, cut the cylinders into lengths of exactly 40 mm. Be sure the cut is perpendicular to the axis of the cylinder. Cut 24 cylinders. • Label six 250 ml beakers as follows: 0.0, 0.1, 0.2, 0.3, 0.4 and 0.6 • Add 100 ml of water to the beaker labeled 0.0. 1 • Calculate the number of grams of sucrose (cane sugar) you will need to add to 100ml of water to make 0.1, 0.2, 0.3, 0.4 and 0.6 molar sucrose solutions. Enter your values in Table 1. Using your calculations prepare the solutions for each of the five remaining labeled beakers. You may use a hot plate and stir bar if necessary to dissolve the sucrose in the water. Table 1 Chemical formula of sucrose, a disaccharide of fructose and glucose _____________ Molecular weight of sucrose __________________________ Molarity (moles/liter) # of grams for 1 liter # of grams for 100ml 0.1 0.2 0.3 0.4 0.6 • • Place 4 potato cylinders into each beaker and incubate them for two hours. After two hours, remove the cylinders and measure their length. Record in the following table. Remember to use significant figures when recording your measurements. Table 2 LENGTH OF POTATO CYLINDER AFTER INCUBATION (mm) - Table Data Molarity of Sucrose 0.0 0.1 0.2 0.3 0.4 0.6 Solutions (moles/liter) Cylinder 1 Cylinder 2 Cylinder 3 Cylinder 4 Mean length Standard deviation % change To calculate the mean and standard deviation see your handout on using the Excel program on the computer. Remember that standard deviation is always recorded as ±. To calculate the percent change in length use the following formula: Mean length after incubation - Original length Original length × 100 All laboratory tables should now write their mean lengths for the potato cylinders at the various molarities on the board. Copy the class data into Table 2. Remember to write your answers with the correct significant figures. 2 Table 3 LENGTH OF POTATO CYLINDERS AFTER INCUBATION (mm) - Class Data Molarities (moles /liter) 0.0 0.1 0.2 0.3 0.4 0.6 Table 1 Table 2 Table 3 Table 4 Table 5 Mean length (class) Standard dev (class) % change (class) Plotting your graph On a separate page make a fourth table to use for construction of your graph. On the x axis, plot the molarity of the sucrose solutions and on the y axis, plot the % change in length. Use the Excel program on the computer to graph your table’s data and the class data. Plot both lines on one graph. (See your Excel handout for plotting a multi-line graph) Now we come to the main purpose of the lab. Question 1: a. What is the osmotic concentration of the potato tissue for your table? b. What is the osmotic concentration of the potato tissue for the class? HINT: This is a case of solving for x (osmotic concentration) when you know y (% change). See your Excel handout on how to solve for x when you know y if you forgot how. Question 2: How do you know what value to use as y in this case? PROBLEMS • How many molecules would be in one mole of sucrose? Show your work for the next two problems or you will get no credit. • How many grams of sucrose would 10 ml of a 0.3M solution contain? • How many molecules of sucrose are contained in 100 ml of a 0.1M sucrose solution? See “Molarity and Avogadro’s Number” handout if you need help. 3