Southgate Schools
advertisement
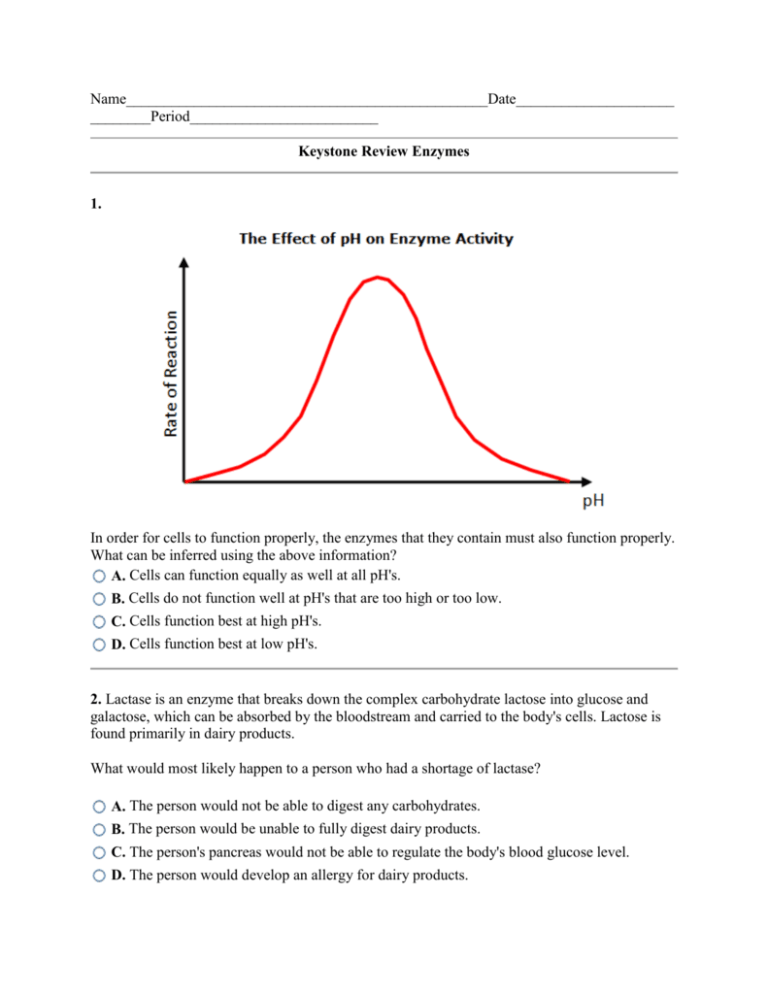
Name________________________________________________Date_____________________ ________Period_________________________ Keystone Review Enzymes 1. In order for cells to function properly, the enzymes that they contain must also function properly. What can be inferred using the above information? A. Cells can function equally as well at all pH's. B. Cells do not function well at pH's that are too high or too low. C. Cells function best at high pH's. D. Cells function best at low pH's. 2. Lactase is an enzyme that breaks down the complex carbohydrate lactose into glucose and galactose, which can be absorbed by the bloodstream and carried to the body's cells. Lactose is found primarily in dairy products. What would most likely happen to a person who had a shortage of lactase? A. The person would not be able to digest any carbohydrates. B. The person would be unable to fully digest dairy products. C. The person's pancreas would not be able to regulate the body's blood glucose level. D. The person would develop an allergy for dairy products. 3. Which of the following describes the role that enzymes play in the process of metabolism? Enzymes store the chemical energy that is used to construct nucleic acids during A. metabolism. B. C. Enzymes carry the genetic instructions required for a cell to initiate metabolism. Enzymes increase the rate of the chemical reactions carried out during metabolism. Enzymes provide the chemical energy that is broken down and released during D. metabolism. 4. Hydrogen peroxide is a substance that is produced as a by-product of many cell processes. However, hydrogen peroxide is toxic to cells. In fact, if cells are unable to break down hydrogen peroxide, they become poisoned and die. The chemical reaction for the breakdown of hydrogen peroxide is shown below. What role does catalase play in this reaction? A. Catalase is a reactant that gets used up in the reaction. B. Catalase is an enzyme that speeds up the reaction. C. Catalase is a product that is created during the reaction. D. Catalase in an enzyme that slows down the reaction. 5. Siamese cats are characterized by light and dark regions of fur. The darker parts occur on the cooler parts of the cat's body because the enzyme that catalyzes the formation of the dark pigment is more active when cool. This example shows that enzymes are affected by . A. temperature B. concentration C. pH D. all of these 6. The graph below shows the relationship between pH and three different digestive enzymes. Pepsin is found in the stomach. Salivary amylase is found in the mouth. And arginase is found in the liver. What does the graph indicate about the relative acidity of these three locations? A. The mouth is more acidic than the stomach, which is more acidic than the liver. B. The stomach is more acidic than the liver, which is more acidic than the mouth. C. The liver is more acidic than the mouth, which is more acidic than the stomach. D. The stomach is more acidic than the mouth, which is more acidic than the liver. 7. A biochemist is attempting to replicate a chemical reaction that commonly takes place in cells. During the chemical reaction, starch is broken down into glucose. What will most likely happen if the biochemist adds an enzyme to the reactants? A. The reaction will take place at a higher temperature. B. The reaction will stop. C. The reaction will speed up. D. The reaction will slow down. 8. The graph below shows the relationship between pH and three different digestive enzymes. Which of the following conclusions can be drawn from this graph? A. Digestive enzymes are most active in an environment with a pH of 7. B. Enzymes operate most efficiently within a certain range of temperatures. C. Each enzyme operates best within a certain pH range. D. Enzymes are only able to operate in acidic environments. 9. Examine the diagram below. If the black line represents a reaction without an enzyme and the red line represents the same reaction with the addition of an enzyme, what can be said of the relationship between the use of an enzyme and the energy of the reaction? A. Less energy is released by a reaction with an enzyme. B. Less energy is released by a reaction without an enzyme. C. More energy is absorbed by a reaction with an enzyme. D. More energy is absorbed by reactions without an enzyme. 10. An enzyme is a biomolecule that increases the rate of a chemical reaction. Which of the following is true about enzymes? I. Enzymes are not consumed during a chemical reaction. II. Enzymes are specific to particular molecules. III. Enzymes increase the energy produced by a chemical reaction. A. I and III only B. I and II only C. II only D. I, II, and III 11. Which of the following enzymes serve as catalysts in metabolism? I. amylase II. lactase III. pepsin IV. polymerase A. I, II, and III only B. I, II, III, and IV C. I and II only D. IV only 12. Examine the diagram of the enzyme catalase below: How does the structure of this enzyme help with its function? A. The enzyme's shape fits its substrate's shape, thus allowing the enzyme to catalyze the appropriate reaction. B. The enzyme's active site can change shape in order to match any substrates in need of a catalyst. C. The enzyme's substrate is a mirror image of the enzyme, thus allowing it to reverse any reactions initiated by the enzyme. D. The enzyme is much larger than its substrate, thus allowing the catalyzed chemical reaction to take place inside the enzyme. 13. An enzyme is a biomolecule that increases the rate of a chemical reaction. An enzyme is a kind of _______. A. inhibitor B. catalyst C. ribosome D. translator 14. Tobin perfomed an experiment in which he mixed varying amounts of enzyme with an excess amount of a certain molecule. He measured the rate of each reaction and recorded his data in the table below. Enzyme Concentration Reaction Rate (millimoles) (micromoles/minute) 0 0 100 0.05 200 0.10 300 0.15 400 0.20 500 0.25 What can Tobin conclude about the relationship between the enzyme concentration and the reaction rate in the presence of excess molecules? A. The experiment is invalid because it was performed in the presence of excess molecules. B. There is an inverse relationship; as the enzyme concentration increases, the reaction rate decreases. C. There is no relationship between the enzyme concentration and the reaction rate. D. There is a direct relationship; as the enzyme concentration increases, the rate of reaction increases. 15. Which of the following can affect the function of a cell? A. high temperature B. high acidity C. low temperature D. all of these 16. All cells in multicellular organisms contain thousands of different kinds of enzymes that are specialized to catalyze different chemical reactions. Given this information, which of the following is most likely to be true? A. All cells contain exactly the same number of enzymes. B. All cells do not contain the same types of enzymes. C. All cells contain exactly the same types of enzymes. D. All cells do not contain enzymes. 17. Many of the biochemical reactions that occur within your cells are catalyzed by enzymes. Which of the following statements is true about most enzyme-assisted reactions? A. Enzymes are quickly used up during the reactions. B. They occur more quickly than reactions without enzymes. C. They can take place only within a ribosome. D. They can take place at any temperature or pH. 18. Catalase is an enzyme that is found in all living tissues. Cells need catalase in order to function properly. Which of the following statements can be inferred using the above information? Cells only function within a specific temperature range because enzymes only function A. within a specific temperature range. Cells only function within a specific pH range because enzymes only function within a B. specific pH range. C. Cells can function equally as well at all pH's because enzymes are effective at all pH's. Cells can function equally as well at all temperatures because enzymes are effective at all D. temperatures. 19. Hydrogen peroxide is a substance that is produced as a by-product of many cell processes. However, hydrogen peroxide is toxic to cells. In fact, if cells are unable to break down hydrogen peroxide, they become poisoned and die. Catalase is an enzyme that is found in all living tissues. This enzyme speeds up the reaction that breaks down hydrogen peroxide into less harmful substances. What substances are formed as a result of this reaction? A. water and oxygen B. water and ozone C. hydrogen peroxide and water D. hydrogen peroxide and oxygen 20. The graph below shows the relationship between pH and three different digestive enzymes. Pepsin is produced by cells lining the stomach. It catalyzes reactions needed to digest certain proteins. After leaving the stomach, food enters the small intestine. The pH of the small intestine is around 7. What would most likely happen to pepsin enzymes that traveled with the food from the stomach to the small intestines? A. The pepsin would begin to digest carbohydrates B. The pepsin would continue to digest proteins in the small intestine. C. The pepsin would turn into the enzyme arginase. D. The pepsin would become inactive. 21. The above graph shows how temperature affects the rate of a reaction that uses the catalase enzyme. What trend is shown by the graph? Between zero and twenty-seven degrees Celsius, the rate of the reaction increases as the A. temperature increases. Between sixty and one hundred degrees Celsius, the rate of the reaction decreases as the B. temperature increases. Between thirty-seven and sixty degrees Celsius, the rate of the reaction increases as the C. temperature increases. Between zero and twenty-seven degrees Celsius, the rate of the reaction decreases as the D. temperature increases. 22. Any factor that changes the shape of an enzyme can affect the enzyme's activity. Which of the following two factors affect an enzyme's operation the most? A. blood glucose level and pH B. temperature and pH C. amount of light and pressure D. amount of light and temperature 23. Enzymes are catalysts. This means that they are able to speed up chemical reactions. Which of the following statements is also true of enzyme catalysts? A. They increase the energy that is released by reactions. B. They increase the energy that must be absorbed by reactions. C. They must be continuously replaced after each catalyzed reaction. D. They are not used up by reactions. 24. When cellular respiration occurs within a cell, carbon dioxide is formed as a waste product. The carbon dioxide is transported out of the cell and absorbed by the blood stream. There, the carbon dioxide undergoes a chemical reaction and forms carbonic acid within the blood. When the carbonic acid reaches the capillaries in the lungs, the enzyme carbonic anhydrase helps reverse the reaction, and the carbonic acid is converted back to carbon dioxide and water. Which of the following would most likely happen if a person's body stopped producing carbonic anhydrase? A. The blood's volume would increase until a hemorrhage occurred. B. The person would begin exhaling carbonic acid instead of carbon dioxide C. The person's breathing rate would slow down. D. The blood's acidity would increase and become toxic. 25. The diagram below represents an enzyme-substrate complex. To which of the following could this complex best be compared? A. lock and key B. salt and pepper C. shoe and sock D. dime and penny 26. The graph below shows the reaction rates of four different chemical reactions. A reaction that is catalyzed by an enzyme usually absorbs less energy and is faster than normal reactions. Which of the reactions was most likely catalyzed by an enzyme? A. Reaction C B. Reaction A C. Reaction B D. Reaction D 27. Homeostasis is the ability of an organism to maintain a relatively stable internal environment. How would an organism's homeostasis be affected if it was not able to produce enzymes? A. Without enzymes, biochemical reactions would cease completely. B. Without enzymes, chemical reactions would not occur quickly enough to sustain life. C. Without enzymes, ribosomes would break down proteins, rather than build them. D. Without enzymes, the temperature inside cells would increase rapidly. 28. The above graph shows how temperature affects the rate of a reaction that uses the catalase enzyme. At what temperatures, approximately, is the enzyme denatured? A. between 37°C and 60°C B. between 60°C and 100°C C. between 27°C and 37°C D. between 0°C and 27°C 29. Examine the following chemical reaction, which is catalyzed by the enzyme catalase: What molecule is catalase breaking down in this reaction? A. There are no molecules in this reaction. B. H2O2 C. O2 D. H2O 30. Suppose that enzyme X catalyzes a reaction that involves the breakdown of a molecule. The product of this reaction is an amino acid. What molecule is the enzyme breaking down? A. a carbohydrate B. a lipid C. a mineral D. a protein Answers 1. B 2. B 3. C 4. B 5. A 6. D 7. C 8. C 9. D 10. B 11. A 12. A 13. B 14. D 15. D 16. B 17. B 18. A 19. A 20. D 21. A 22. B 23. D 24. D 25. A 26. C 27. B 28. B 29. B 30. D Explanations 1. Enzymes can only function within a narrow pH range. If the pH is too high (too basic) or too low (too acidic), the enzyme changes its shape and is no longer effective. Since enzymes play an important role in nearly all cellular processes, cells can only function within a specific pH range, too. Cells do not function well at pH's that are too high or too low. 2. Many people are lactose intolerant, meaning that they have difficulty digesting food that contains large amounts of lactose. This occurs because a person's body does not produce enough lactase enzyme, which is the enzyme needed to quickly breakdown lactose. 3. Enzymes increase the rate of the chemical reactions carried out during metabolism by lowering the activation energy necessary to initiate these reactions. Without enzymes catalyzing the chemical reactions that are performed during metabolism, cells would not be able to perform metabolism quickly enough to support life. 4. Catalase is an enzyme that is found in all living tissues. This enzyme speeds up the reaction that breaks down hydrogen peroxide into water and oxygen. 5. Although enzymes are also affected by the pH and the concentration of the enzyme and substrate in their environment, this specific example shows that enzymes are affected by temperature. 6. Enzymes are well-adapted to function optimally in the places where they are found in the body. Knowing this, you can ascertain from the graph that the stomach has an average pH of 2, the mouth has an average pH of 7, and the liver has an average pH of 9.5. 7. Enzymes are substances that cause chemical reactions to occur faster. 8. All enzymes have a range of pH at which they are active, as well as an optimal pH at which they are most active. For example, salivary amylase is active in a pH range of 4.7 to 9.0, but it operates best in an environment with a pH of 6.9. 9. Many chemical reactions need energy to get them started. This energy is known as the activation energy. Enzymes help catalyze, or speed up, these chemical reactions by lowering the amount of activation energy needed for the reactions to proceed. Reactions that do not use an enzyme require more energy. 10. Enzymes are catalysts that increase the rate of chemical reactions by decreasing the amount of energy needed to activate the chemical reaction. Enzymes are not consumed during a chemical reaction and, therefore, once an enzyme has finished catalyzing one reaction, it can move on and catalyze another reaction. Enzymes are also specific to particular molecules based on their shape and size. Enzymes do not, however, increase the amount of energy produced by a chemical reaction. Only I and II are characteristics of enzymes. 11. Amylase, lactase, and pepsin play important roles in catalyzing the reactions that occur during metabolism. Amylase is an enzyme that aids in the digestion of carbohydrates. It is present in the saliva and pancreas of humans and catalyzes the breakdown of starches into smaller carbohydrates. Lactase is an enzyme that is found primarily in the small intestine, liver, and kidney. Lactase catalyzes the breakdown of lactose into simple sugars, such as glucose. Pepsin is an enzyme that aids in the digestion of proteins, such as those found in meat and eggs. Pepsin is primarily found in the gastric juice of the stomach. Only I, II, and III serve as catalysts in metabolism. Polymerase is an enzyme that plays an important role in the polymerization of new DNA and RNA molecules during DNA replication and transcription. 12. The shape of an enzyme determines how it works. Most enzymes have a surface with one or more deep folds. The folds make pockets, which are called active sites. The active sites match folds in the substrate's surface. Once an enzyme binds to a substrate, the amount of energy needed to start a chemical reaction with the substrate is reduced. 13. Catalysts accelerate the rate of a chemical reaction. Chemical reactions that occur in living systems can be catalyzed by protein molecules called enzymes, which are a type of catalyst. 14. In the presence of excess molecules, there is a direct relationship between the enzyme concentration and the reaction rate. As the enzyme concentration increases, the reaction rate also increases. If this experiment were not performed with excess molecules, the results would initially be the same, but the reaction rate would eventually plateau once all the molecules were used up. 15. Most cell function best within a narrow range of temperature and acidity. At very low temperatures, reaction rates are too slow. High temperatures and/or extremes of acidity can irreversibly change the structure of most protein molecules. Even small changes in acidity can alter the molecules and how they interact. 16. In multicellular organisms, cells are specialized to perform specific functions in different areas of the body. Since cells perform different functions, they require different enzymes. Thus, all cells do not contain the same types of enzymes. 17. Most enzyme-assisted reactions occur more quickly than reactions without enzymes. Enzymes lower the energy that the reaction needs to begin, thus increasing the rate of the reaction. 18. The graph shows that catalase only functions within a specific temperature range. If the temperature is too high, catalase changes its shape and is no longer effective. Since catalase plays an important role in all cells, cells can only function within a specific temperature range. Although it is also true that cells can only function within a specific pH range, this fact cannot be inferred from the given information. 19. According to the chemical equation, water and oxygen are produced when hydrogen peroxide is broken down. The enzyme catalase speeds up this reaction. 20. As the graph shows, pepsin only remains active up to a pH of about 4.8. If the pH is any higher than this, the pepsin's folds will become so distorted that its active site will no longer function. Thus, pepsin is only able to catalyze the digestion of proteins in the highly acidic environment of the stomach. 21. In general, between zero and twenty-seven degrees Celsius, the rate of the reaction increases as the temperature increases. Then, between thirty-seven and sixty degrees Celsius, the rate of the reaction decreases as the temperature increases. Finally, between sixty and one hundred degrees Celsius, no reaction occurs; the rate of the reaction equals 0 ml/s. At these temperatures, the enzyme is denatured. 22. Most enzymes are only active within certain temperature and pH ranges. 23. Imagine that you need to turn a nut onto the end of a bolt. You could do it by hand, but it would be faster and require less energy if you used a power tool instead. Then, once you were done with that bolt, you could use the same power tool on a different bolt. An enzyme is like a power tool because, by definition, catalysts speed up chemical reactions without being used up by the reaction. So, once an enzyme has finished catalyzing one reaction, it can move on and catalyze another reaction, because it is not used up by the reaction. 24. Without carbonic anhydrase, the reaction that causes carbonic acid to become carbon dioxide and water would not take place quickly enough for the person to exhale enough carbon dioxide. The carbon dioxide, in the form of carbonic acid, would build up in the bloodstream, causing the blood pH to rapidly decrease to toxic levels. 25. Enzymes have very unique shapes that allow them to bind to specific molecules and catalyze specific reactions. Thus, an enzyme-substrate complex can best be compared to a lock and key. 26. Enzymes reduce the activation energy (the energy reactants need to absorb) of chemical reactions. This allows the reaction to speed up. Reaction B was most likely catalyzed by an enzyme because it took a low amount of energy to start and the reaction was fast compared to Reactions C and D. 27. Most chemical reactions occur very slowly at low temperatures, including the temperatures found inside the bodies of most animals. Without enzymes, most of the vital chemical reactions that take place inside cells would occur so slowly that the organism would die before the necessary products were achieved. Enzymes allow the reactions to speed up to the rate necessary to keep the organism alive. 28. The enzyme catalase is most effective when the reaction rate is at its peak (1 ml/s). This occurs between 27°C and 37°C, approximately. Between approximately 60°C and 100° C, the rate of the reaction is equal to 0 ml/s. At these temperatures, the enzyme catalase is denatured. That is, the structure of the enzyme changes, so it is no longer able to catalyze reactions. 29. In the given reaction, hydrogen peroxide (H2O2) was broken down into water and oxygen in the presence of catalase. 30. Amino acids are the building blocks of proteins. If the enzyme catalyzes a reaction that involves the breakdown of a protein, amino acids would result. Amino acids would not result if the molecule was a carbohydrate, lipid, or mineral.






