Sonntag, Borgnakke and van Wylen 4.28 A car drives for half an
advertisement

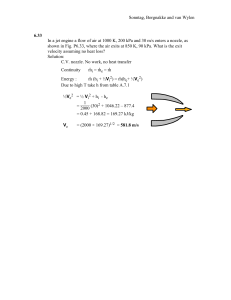
Sonntag, Borgnakke and van Wylen 4.28 A car drives for half an hour at constant speed and uses 30 MJ over a distance of 40 km. What was the traction force to the road and its speed? Solution: We need to relate the work to the force and distance W=¶ ´F dx = F x W 30 000 000 J F = x = 40 000 m = 750 N L 40 km km 1000 m V = t = 0.5 h = 80 h = 80 3600 s = 22.2 ms1 Sonntag, Borgnakke and van Wylen 4.33 A 400-L tank A, see figure P4.33, contains argon gas at 250 kPa, 30oC. Cylinder B, having a frictionless piston of such mass that a pressure of 150 kPa will float it, is initially empty. The valve is opened and argon flows into B and eventually reaches a uniform state of 150 kPa, 30oC throughout. What is the work done by the argon? Solution: Take C.V. as all the argon in both A and B. Boundary movement work done in cylinder B against constant external pressure of 150 kPa. Argon is an ideal gas, so write out that the mass and temperature at state 1 and 2 are the same PA1VA = mARTA1 = mART2 = P2( VA + VB2) => VB2 = 2 250 u 0.4 - 0.4 = 0.2667 m3 150 3 ´ PextdV = Pext(VB2 - VB1) = 150 kPa (0.2667 - 0) m = 40 kJ 1W2 = ¶ 1 Sonntag, Borgnakke and van Wylen 4.42 A piston cylinder contains 1 kg of liquid water at 20oC and 300 kPa. There is a linear spring mounted on the piston such that when the water is heated the pressure reaches 3 MPa with a volume of 0.1 m3. a) Find the final temperature b) Plot the process in a P-v diagram. c) Find the work in the process. Solution: Take CV as the water. This is a constant mass: m2 = m1 = m ; State 1: Compressed liquid, take saturated liquid at same temperature. B.1.1: v1 = vf(20) = 0.001002 m3/kg, State 2: v2 = V2/m = 0.1/1 = 0.1 m3/kg and P = 3000 kPa from B.1.3 => Superheated vapor close to T = 400oC Interpolate: T2 = 404oC Work is done while piston moves at linearly varying pressure, so we get: 1 1W2 = ³ P dV = area = Pavg (V2 V1) = 2 (P1 + P2)(V2 - V1) = 0.5 (300 + 3000)(0.1 0.001) = 163.35 kJ P C.P. T 2 C.P. 2 300 kPa 1 300 T 20 v 1 v Sonntag, Borgnakke and van Wylen 4.46 Consider the problem of inflating the helium balloon, as described in problem 3.79. For a control volume that consists of the helium inside the balloon determine the work done during the filling process when the diameter changes from 1 m to 4 m. Solution : Inflation at constant P = P0 = 100 kPa to D1 = 1 m, then P = P0 + C ( D* -1 - D* -2 ), D* = D / D1, to D2 = 4 m, P2 = 400 kPa, from which we find the constant C as: 400 = 100 + C[ (1/4) - (1/4)2 ] => C = 1600 kPa S The volumes are: V = 6 D3 => V1 = 0.5236 m3; V2 = 33.51 m3 2 WCV = ´ ¶ PdV 1 2 * -1 - D* -2)dV = P0(V2 - V1) + ´ ¶ C(D 1 S V = 6 D3, S S dV = 2 D2 dD = 2 D13 D* 2 dD* D2*=4 WCV = P0(V2 - V1) + 3CV1 ´ ¶ (D*-1)dD* D1*=1 4 D2* 2 - D1* 2 * * = P0(V2 - V1) + 3CV1[ (D D ) ] 2 1 2 1 16-1 = 100u33.51 – 0.5236) + 3u1600u0.5236 [ 2 – (4–1)] = 14 608 kJ Sonntag, Borgnakke and van Wylen 4.48 The piston/cylinder shown in Fig. P4.48 contains carbon dioxide at 300 kPa, 100qC with a volume of 0.2 m3. Mass is added at such a rate that the gas compresses according to the relation PV1.2 constant to a final temperature of 200qC. Determine the work done during the process. Solution: From Eq. 4.4 for the polytopic process PVn = const ( n =/ 1 ) 2 P2V2 - P1V1 W = PdV = ´ 1 2 ¶ 1-n 1 Assuming ideal gas, PV = mRT mR(T2 - T1) , 1W2 = 1-n P1V1 300 u 0.2 kPa m3 But mR = T = 373.15 K = 0.1608 kJ/K 1 1W2 = 0.1608(473.2 - 373.2) kJ K 1 - 1.2 K = -80.4 kJ Sonntag, Borgnakke and van Wylen 4.63 A piston/cylinder assembly (Fig. P4.63) has 1 kg of R-134a at state 1 with 110qC, 600 kPa, and is then brought to saturated vapor, state 2, by cooling while the piston is locked with a pin. Now the piston is balanced with an additional constant force and the pin is removed. The cooling continues to a state 3 where the R-134a is saturated liquid. Show the processes in a P-V diagram and find the work in each of the two steps, 1 to 2 and 2 to 3. Solution : CV R-134a This is a control mass. Properties from table B.5.1 and 5.2 State 1: (T,P) B.5.2 => v = 0.04943 m3/kg State 2: given by fixed volume v2 = v1 and x2 = 1.0 v2 = v1 = vg = 0.04943 m3/kg State 3 reached at constant P (F = constant) so from B.5.1 => T = 10qC v3 = vf = 0.000794 m3/kg P 1 3 cb 2 V Since no volume change from 1 to 2 => 1W2 = 0 Constant pressure 2W3 = ³P dV = P(V3 -V2) = mP(v3 -v2) = 415.8 (0.000794 - 0.04943) 1 = -20.22 kJ Sonntag, Borgnakke and van Wylen 4.73 A film of ethanol at 20qC has a surface tension of 22.3 mN/m and is maintained on a wire frame as shown in Fig. P4.73. Consider the film with two surfaces as a control mass and find the work done when the wire is moved 10 mm to make the film 20 u 40 mm. Solution : Assume a free surface on both sides of the frame, i.e., there are two surfaces 20u30 mm -3 -6 W = ´ ¶ S dA = 22.3u10 u2(800 600)u10 = 8.92u10-6 J = -8.92 PJ Sonntag, Borgnakke and van Wylen 4.94 A torque of 650 Nm rotates a shaft of diameter 0.25 m with Z = 50 rad/s. What are the shaft surface speed and the transmitted power? Solution: V = Zr = ZD/2 = 50 u0.25 / 2 = 6.25 m/s Power = TZ = 650 u 50 Nm/s = 32 500 W = 32.5 kW Sonntag, Borgnakke and van Wylen 4.97 A water-heater is covered up with insulation boards over a total surface area of 3 m2. The inside board surface is at 75qC and the outside surface is at 20qC and the board material has a conductivity of 0.08 W/m K. How thick a board should it be to limit the heat transfer loss to 200 W ? Solution : Steady state conduction through a single layer board. . . 'T Q cond = k A 'x k $'7Q 'x 75 20 'x = 0.08 u3u 200 = 0.066m