Cell Walls and Plant Anatomy
advertisement
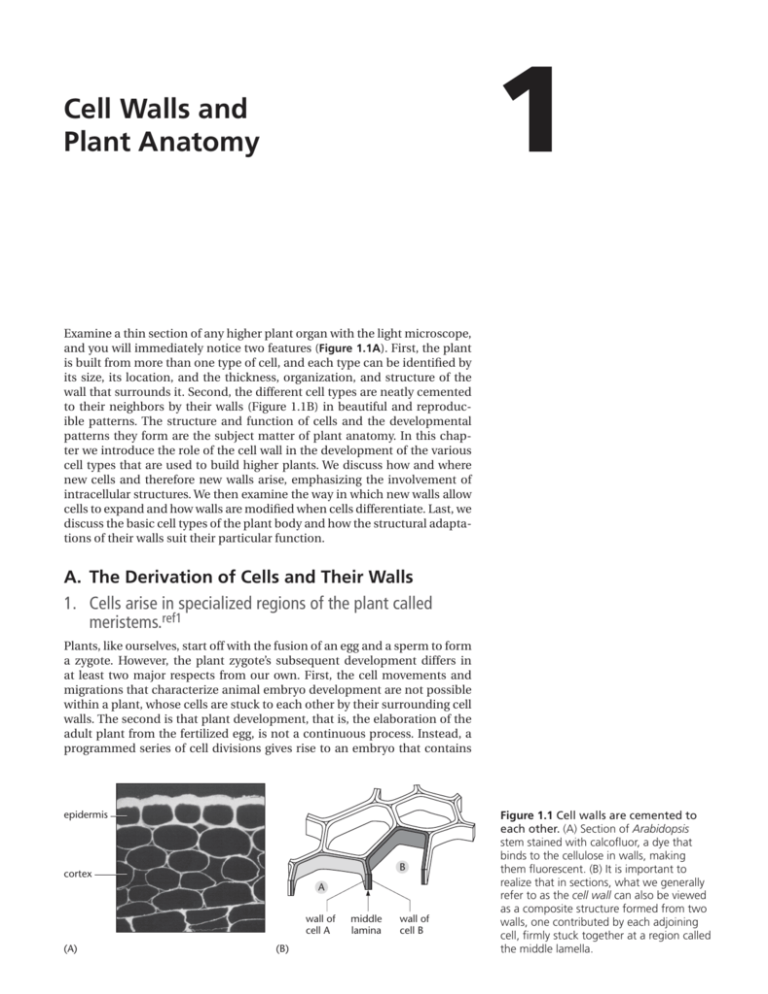
1 Cell Walls and Plant Anatomy Examine a thin section of any higher plant organ with the light microscope, and you will immediately notice two features (Figure 1.1A). First, the plant is built from more than one type of cell, and each type can be identified by its size, its location, and the thickness, organization, and structure of the wall that surrounds it. Second, the different cell types are neatly cemented to their neighbors by their walls (Figure 1.1B) in beautiful and reproducible patterns. The structure and function of cells and the developmental patterns they form are the subject matter of plant anatomy. In this chapter we introduce the role of the cell wall in the development of the various cell types that are used to build higher plants. We discuss how and where new cells and therefore new walls arise, emphasizing the involvement of intracellular structures. We then examine the way in which new walls allow cells to expand and how walls are modified when cells differentiate. Last, we discuss the basic cell types of the plant body and how the structural adaptations of their walls suit their particular function. A. The Derivation of Cells and Their Walls 1. Cells arise in specialized regions of the plant called meristems.ref1 Plants, like ourselves, start off with the fusion of an egg and a sperm to form a zygote. However, the plant zygote’s subsequent development differs in at least two major respects from our own. First, the cell movements and migrations that characterize animal embryo development are not possible within a plant, whose cells are stuck to each other by their surrounding cell walls. The second is that plant development, that is, the elaboration of the adult plant from the fertilized egg, is not a continuous process. Instead, a programmed series of cell divisions gives rise to an embryo that contains epidermis B cortex A wall of cell A (A) (B) middle lamina wall of cell B Figure 1.1 Cell walls are cemented to each other. (A) Section of Arabidopsis stem stained with calcofluor, a dye that binds to the cellulose in walls, making them fluorescent. (B) It is important to realize that in sections, what we generally refer to as the cell wall can also be viewed as a composite structure formed from two walls, one contributed by each adjoining cell, firmly stuck together at a region called the middle lamella. 2 Chapter 1 Cell Walls and Plant Anatomy shoot pole (A) shoot apical meristem 100 mm root pole embryo (C) (B) 100 mm 100 mm Figure 1.2 Apical meristems derive from the root and shoot poles of the embryo. (A) A scanning electron micrograph shows the shoot apex with two sequentially emerging leaf primordia, seen here as lateral swellings on either side of the domed apical meristem. (B) A thin section of a similar apex shows that the youngest leaf primordium arises from a small group of cells (about 100) in the outer four or five layers of cells. (C) The root meristem and root cap of a corn root, showing the orderly files of cells produced. (A and B, from R.S. Poethig and I.M. Sussex, Planta 165:158–169, 1985. C, from P.H. Raven, R.F. Evert, and S.E. Eichhorn, Biology of Plants, 4th ed. New York: Worth, 1986.) pcw 1A1.1/1.02 a shoot pole, a root pole, and either one or more seed leaves; and at this point the embryo’s development is commonly arrested in a seed. Within the maternal ovule tissue of the seed, the embryo will remain dormant until seed dispersal and suitable environmental conditions permit it to germinate and grow. The young plant seedling will grow by a coordinated combination of cell enlargement and the production of new cells. However, cell proliferation does not occur evenly throughout the plant body. Following germination, two specialized groups of cells at opposite ends of the seedling axis, the shoot and root poles, begin to proliferate actively. Although cell divisions can occur elsewhere in the plant, these two groups of cells, now called the shoot apical meristem and the root meristem, respectively, will become a major source of new cells in the plant. They both perpetuate themselves and also give rise to new meristems in an iterative process that produces the characteristic structure of the mature plant, including structures such as leaves and flowers (Figure 1.2). Other groups of dividing cells can arise in established tissues, where they are called lateral meristems. These give rise to lateral roots and, through the cambium and cork cambium contribute to growth in thickness of the plant. A simple overview of higher plant anatomy is discussed later (see Panel 1.1). Meristems themselves have a relatively defined, species-specific structure, but all are composed of small, densely cytoplasmic cells, generally less than 10 mm in diameter and having thin cell walls (Figure 1.3). Both shoot apical and root meristems contain two distinct populations of cells. A small subset of cells, called initials, or stem cells, divide surely but slowly. When The Derivation of Cells and Their Walls nucleus vacuole 3 Figure 1.3 Meristematic cells. Typical meristematic plant cells are small, with very small vacuoles and thin cell walls. This example is from a shoot apical meristem. (Courtesy of Ichirou Karahara.) cell wall 5 mm they divide, they produce one daughter cell that remains a stem cell and another daughter cell that enters the cell population of the rest of the meristem. These cells may divide further, but eventually they leave the meristem. It is largely within these populations of dividing cells that new primary walls are produced. 2. Walls originate in dividing cells.ref2 PCW 1A1.2/1.3 If we consider a single cell within an apical meristem, it is tempting to think that its surrounding wall arose in a single process of wall deposition around the living protoplast. But that would be to ignore the course of development. In fact, every facet of its polyhedral wall was laid down at a different time, namely, when that cell was cut off from one of its immediate neighbors by a cross wall during cell division. In some cases this might be a recent event, but in others the wall might derive, by growth, from one laid down during a cell division much earlier, for example, in the embryo. Each wall is thus a patchwork coat, with each patch reflecting events that happened at different times in the cell’s development. Each cell, consequently, carries within its wall a historical relationship with all of its neighbors (Figure 1.4). 1 2 1 3 2 2 3 4 4 Figure 1.4 Sequential cell divisions create “walls” with different histories. Schematic diagram, showing how a sequence of cell divisions creates a series of intersecting cell walls of different ages (shaded gray). The resultant final cell pattern is shown on the right with the wall of the central cell shown in black. This is usually interpreted as a single cell wall, but developmentally it has been made by the consecutive addition of different cell plates that eventually intersect with each other. A particular wall (for example, wall 1) has a history in common with the walls of adjacent cells with which it shares ancestry, rather than necessarily with wall 3, for example, which has only recently been cut and pasted into it. This example emphasizes the problem of whether a “wall” stops at the middle lamella or is a single partition that includes the contribution from two neighboring cells. 4 Chapter 1 Cell Walls and Plant Anatomy 25 mm Figure 1.5 The origin of a new cell wall. Sequential light micrographs of a dividing stamen hair cell. The elapsed time in minutes is shown at the bottom left corner of each photograph. By 42 minutes the nucleus has undergone mitosis, cytokinesis is under way, and the early cell plate can be seen between the two daughter nuclei. This extends rapidly outward until it reaches and fuses with the mother cell wall. (Courtesy of Peter Hepler.) With very few exceptions, new cross-walls arise only when cells divide and, following nuclear division or mitosis, they partition the protoplast in a process called cytokinesis. Plant cytokinesis is an inside-outward event that starts with the formation of a small, disklike wall, or cell plate, inside the cell, 1A2.2/1.05 between the two daughter nuclei. This cell plate extends radially outPCW ward until it finally reaches and fuses with the mother cell wall, thus cutting the cell in two (Figure 1.5). The structure that assembles this cell plate is called the phragmoplast; it originates most commonly from the microtubules of the two half spindles of the mitotic apparatus and has a disklike structure, with the plus ends of the microtubules on either side ending in a plane at right angles to the spindle axis. Associated with this structure are actin filaments, closely aligned with the microtubules. The phragmoplast is surrounded by numerous Golgi stacks, which produce vesicles that are transported along the microtubules to the center of the phragmoplast. Here, the vesicles fuse to form a membrane-enclosed disk, the cell plate (Figure 1.6). The vesicles carry pectic polysaccharides, xyloglucan, and proteins to the cell plate; this subject is discussed in greater detail in Chapter 4. The polysaccharide callose, a b1-3 glucan, can be detected in the plate at an early stage, but the cellulose, characteristic of the final wall, is not deposited until the latest stages of cell plate formation. Intact phragmoplasts that can continue to make wall material for the cell plate can be isolated from synchronized cells in suspension culture (Figure 1.7). For complete partition, even in a small meristematic cell, the cell plate must be extended at its edge until it finally makes contact with the mother cell wall to produce a cross-wall. The phragmoplast achieves this by continuously disassembling the microtubules at its center and reassembling them at the edge of the growing cell plate, where they function in guiding new vesicles to fuse with and extend its margin (Figure 1.8). At about the time the cell plate finally reaches the mother cell wall and separates the two daughter cells, callose is removed and cellulose deposition begins on each face of the plate, allowing us to finally distinguish two separate “walls” within the partition, one being made by and belonging to one daughter cell and one being made by and belonging to the other. 3. Plant organ development depends on precise control of the plane of cell division and of cell expansion.ref3 A single isolated plant cell grown in culture can successfully divide and expand or elongate. If its cell wall is removed, however, the remaining protoplast, although living, can neither divide nor elongate unless it regenerates its wall. Correspondingly, the walled cell cannot divide or elongate unless its cytoskeleton is both intact and functional. The proliferation and growth of plant cells depends on a functional interaction between elements inside the plasma membrane—in particular the cytoskeleton—and the cell wall outside the plasma membrane. However, during tissue development, these The Derivation of Cells and Their Walls cell A in telophase 5 cell B in cytokinesis new cell plate 5 mm Figure 1.6 The cell plate and dividing cells. This electron micrograph is of a thin longitudinal section through a maize root meristem. The long axis of the root runs from left to right. The large central cell has already divided once to form two new cells, A and B, with a thin young primary wall separating them. The two daughter cells are each now dividing again. Cell A is in late telophase, and the two separated sets of chromosomes are visible. Cell B has reached the next stage of cell division, and between the two re-forming nuclei an early stage of vesicle accumulation and cell plate formation can be seen. (Courtesy of Adrian Turner.) cell-autonomous activities are tightly coupled with those of neighboring cells to generate the precise and reproducibly structured organs we find in the mature plant. There are additional constraints on the generation of plant form. We have already mentioned that the cells of plants are immobile and that, with very few exceptions, adjacent cells are firmly stuck together and are thus prevented from expanding at different rates and “slipping” relative to one another. Thus, local cell-cell interactions are central to coordination pcwthe 1A2.3/1.06 and control of plant growth. We are now in a position to appreciate that, in many cases, it is the accurate placing of new cross-walls during cell division, followed by the subsequent controlled expansion and/or elongation of the resultant cells, that has (A) (B) 10 mm Figure 1.7 Polysaccharide synthesis in isolated phragmoplasts. Radioactive precursors are incorporated into wall polysaccharides by phragmoplasts isolated from tobacco cells in culture. These retain all the cytoplasmic organelles usually associated with the phragmoplast, including Golgi stacks. (A) When the phragmoplasts are fed with radioactive UDP-xylose, this marker is incorporated into matrix polysaccharides in the Golgi stacks surrounding the cell plate (arrows) and can be revealed by autoradiography as black silver grains. (Golgi stacks cannot transport the product to the cell plate within the isolated phragmoplast.) (B) When phragmoplasts are fed with labeled UDP-glucose, the label is incorporated into callose by enzymes located in the membrane of the cell plate. Here the silver grains are located directly over the cell plate (arrows). (From T. Kakimoto and H. Shibaoka, Plant Cell Physiol. 33:353–361, 1992.) 6 Chapter 1 Cell Walls and Plant Anatomy (A) Figure 1.8 The phragmoplast and cell plate. (A) Sequence of events in phragmoplast formation. (B) A light micrograph of a plant cell entering cytokinesis. The cell has been stained to show both the microtubules and the two sets of daughter chromosomes. The clear region where the new cell plate is being assembled is indicated by the arrowheads. (B, courtesy of Andrew Bajer.) (B) 50 mm a major influence on plant organ development. For example, in the first early divisions of the zygote of the model plant Arabidopsis (Figure 1.9), the precise sequential placing of the first cross-walls that produces the precise pattern of cells in the embryo appears to be essential for subsequent development. Mutations that affect these early embryonic division planes produce severely deformed or nonviable embryos. Later in development, common patterns of division and expansion often emerge. Thus, meristems often contain cells that reiterate transverse divisions to produce extended files of cells (Figure 1.10). The continuous cell proliferation activity of meristems creates a population pcw 1A2.5/1.08 of cells that will divide less frequently, will expand, and will eventually differentiate into mature cell types. Both the spatial and temporal controls over the plant cell cycle and the spatial and temporal controls over cell expansion have to be tight and, more important, coordinated with neighboring cells. Although such controls are likely to include mechanical constraints, environmental and physiological cues, and the action of conventional plant growth factors, the detailed molecular machinery through which they work has not been fully elucidated. Figure 1.9 Early sequence of divisions in the Arabidopsis zygote. The fertilized egg (A) divides transversely to form an upper cell (gray) that will form the embryo proper, and a lower cell (white) that will form the suspensor and part of the root meristem (B). The embryo proper goes through a series of stereotyped divisions (C–F) that include, for example, some periclinal divisions (E) that distinguish an outer layer of cells that will form the entire shoot epidermis (dark gray). In some other species, early embryonic divisions are much less organized. Despite our emphasis so far on the pattern of sequential divisions, it should be noted that plant structure is characterized by flexibility and plasticity, and it is the interaction between the separate processes of cell proliferation and cell expansion that generates plant form. In some cases, properties of the whole organ can override or entrain the contributions of division and expansion at the cellular level, as in the maize mutant tangled. In a normal maize plant, the leaf epidermis has a highly ordered pattern of longitudinal and transverse divisions that contribute to the width and length, respectively, of the leaf. By contrast, in the tangled mutant, the leaf epidermis has large numbers of cells with aberrant division planes. Despite the rather (A) (B) (C) (D) (E) (F) The Derivation of Cells and Their Walls epidermis cortex endodermis Figure 1.10 Root meristems produce organized files of cells. (A) A scanning electron micrograph of an Arabidopsis root tip showing the longitudinal files of cells produced by transverse divisions of the epidermal cells. The diameter of this root is exactly comparable to a human hair. (B) A longitudinal section of a similar root shows that the files of cells can be traced back to their origins within the meristem. The epidermis in this section is largely covered by cells of the root cap. (A, courtesy of Paul Linstead.) root cap (B) (A) 50 mm muddled pattern of resulting cell walls, the overall shape of the mutant leaf is remarkably similar to that of the wild type (Figure 1.11). The TANGLED gene is required for the proper positioning of the cytoskeletal arrays involved in the formation and placement of new cell plates, which we discuss next. The way in which larger-scale structures and their growth patterns can affect cell division and expansion patterns remains obscure. (A) 7 (B) pcw 1A3.2/1.10 Figure 1.11 Division plane control. Scanning electron micrographs of the surface of maize leaf primordia. (A) Wild type and (B) the mutant tangled. Despite the disorganized division planes, the mature leaf that finally develops looks remarkably normal in shape. (Courtesy of Laurie Smith.) 8 Chapter 1 Cell Walls and Plant Anatomy forming mitotic spindle nucleus preprophase band of microtubules (A) (B) Figure 1.12 The preprophase band. In these living tobacco cells in culture, the microtubules are stained because the cell is expressing a green fluorescent protein fused to a microtubule-binding protein. The microtubules in the dividing cell gradually accumulate in the preprophase band (A–C) and also begin to form the mitotic spindle (C). Cortical microtubules can be seen in the nondividing cells. (D) The preprophase band encircles the cell, defining the future division plane. (A–C, courtesy of Henrik Buschmann.) (C) (D) 4. Cytoskeletal elements predict the position of the new cross-wall before the cell divides.ref4 Thin sections of healthy plant tissue show clear, reproducible, and speciesspecific patterns of cells. This consistent anatomy strongly indicates that, during the cell divisions that give rise to the tissue, the positioning of new cross-walls is an extremely accurate process. What mechanisms exist within plant tissues to ensure such precision in cell partitioning? In almost every PCW 1A4.1/1.12 case, shortly after DNA replication has finished, cytoskeletal elements rearrange themselves within the cell into a structure just inside the plasma membrane that predicts the site where the new cell plate will later meet the mother cell wall. The most obvious feature of this structure is a tight bundle of microtubules lying just beneath the plasma membrane that has been called the preprophase band (Figure 1.12). The ability of this band to prefigure the exact future division plane is true for both symmetric and asymmetric cell divisions (Figure 1.13). The nucleus at the early prophase stage of mitosis, with its condensing chromosomes, typically lies at the center of the plane defined by the preprophase band and appears to be held there by cytoplasmic strands that also contain both microtubules and actin filaments. One might think of the whole structure as rather like a bicycle wheel in which the rim represents the preprophase band, the hub represents the nucleus, and the spokes represent the microtubules and actin filaments radiating from the nucleus to the preprophase band (Figure 1.14 and Figure 1.15). subsidiary cell Figure 1.13 A preprophase band predicts a future asymmetric cell division. In the epidermis of a grass leaf (rye) a series of divisions gives rise to a mature stoma flanked by two guard cells and two subsidiary cells. (A) The subsidiary cells arise by an asymmetric division in the subsidiary cell mother cell (B) that is predicted by a curved preprophase band. (C) The immunofluorescence image shows the microtubules of the preprophase band revealed by an antibody to tubulin. (C, from S.-O. Cho and S.M. Wick, J. Cell Sci. 92:581–594, 1989.) 20 mm (A) stomatal guard cell preprophase band in subsidiary cell mother cell (B) (C) 10 mm The Derivation of Cells and Their Walls 9 Figure 1.14 Microtubules in the preprophase band. These electron micrographs of a thin section of a leaf epidermal cell from sugar cane show the preprophase band of microtubules just beneath the plasma membrane. The cell in this case is part of the stomatal complex and is dividing asymmetrically to produce a smaller daughter cell that will form the stomatal subsidiary cell. (Courtesy of C.H. Busby.) It may seem paradoxical that the microtubules of the preprophase band, which appear to predict so accurately the position of the future cross-wall, actually disappear as the cell enters mitosis. The nature of the “molecular memory” that remains at thePCW cell1A4.3/1.14 surface appears to be produced by remodeling of the plasma membrane in the region of the preprophase band by endocytic vesicles that remove selected membrane proteins. The significance of this is explored in the next section. The signals or cues that are used by a cell to position the preprophase band are largely unknown. However, one signal used by many cells to determine the division plane is mechanical stress. Artificially applied pressure on living plant tissue commonly triggers new cell divisions in which the new crosswalls are oriented at right angles to the applied pressure. To what extent such cues operate to determine the position of preprophase bands during normal development is not known. 5. Actin filaments help to position new cross-walls.ref5 We have seen how placement of the new cross-wall during cell division is faithfully predicted by the position of the preprophase band of microtubules. Both environmental cues—for example, physical pressure—and local factors can influence cross-wall positioning. In particular, the shape of the cell and the local cellular geometry of neighboring cells influence the decision about where the preprophase band, and hence the future cross-wall, will be located. For example, long cells tend to divide with the new cross-wall at right angles to the long axis of the cell. A cross-wall in one cell also tends to inhibit the placing of a new cross-wall directly opposite in the neighboring cell. In other words, three-way junctions (places at which three cells contact each other) are strongly favored over four-way junctions (Figure 1.16). In groups of cells in which the final pattern of divisions is highly repeatable, such as the stomatal divisions in Figure 1.13, the extending cell plate meets the mother cell wall at the predetermined site with an accuracy of less than 1 mm, and we must consider now how this relates to the earlier positioning of the preprophase band. Following the disappearance of the microtubules from the preprophase band, the actin filaments that remain appear to have several major roles in subsequent events. First, an actin-free zone persists in place of the preprophase band, probably originating by local endocytosis removing membrane-associated actin-binding proteins. This in some way marks that cortical region of the cell with which the new cross-wall will fuse. Groups of actin filaments, extending from the surface of the nucleus to a region at each end of the cell, create a force on the developing spindle that helps align it perpendicular to the plane of the preprophase band (Figure 1.17A–C). This ensures that, following chromosome separation, the developing phragmo- 20 mm Figure 1.15 A preprophase band in a large dividing vacuolate epidermal cell. The cell is fluorescently labeled with an antibody to tubulin and the original image is three-dimensional, made by combining PCW 1A4.4/1.15 a stack of separate optical sections. The nucleus, in the center of the cell, is clearly connected to the cortical preprophase band of microtubules by radial bundles of microtubules. (From C.W. Lloyd, ed., Cytoskeletal Basis of Plant Growth and Form. London: Academic Press, pp. 245–257, 1991.) 10 Chapter 1 (A) (B) (C) (D) (E) (F) pcw 1A5.1/1.16b-f Cell Walls and Plant Anatomy 30 mm Figure 1.16 Cell division planes and the preference for threeway junctions. (A) A scanning electron micrograph of the surface of an oilseed rape embryo reveals that junctions are almost invariably between three cells rather than four or more. (B–F) These preferred Y-shaped, three-way junctions arise because new cell walls that are inserted during cell division generally avoid a preexisting junction as is shown below schematically. (B, C) A new division wall is inserted transversely in the left-hand cell of two young expanding cells. (D) As the cells expand it is thought that the new wall lags behind and the common wall buckles to produce a Y-shaped three-way junction. When the right-hand cell divides, the preprophase band avoids this vertex, and when the new division wall is complete (E), it is staggered with respect to the earlier wall. (F) This junction, too, will eventually become Y-shaped. Similar, and well understood, geometries occur in rafts of soap bubbles, but the molecular basis for selecting the preprophase band site, although influenced in part by geometry and mechanical forces, is by no means completely understood. (A, courtesy of Lloyd Peto and Kim Findlay.) plast, and therefore the developing cell plate, is in the plane defined by the preprophase band. Second, the groups of radial actin filaments persist and become attached to the edge of the growing cell plate. These filaments exert tension and guide the extending plate accurately to its final cortical position where the actin-free zone was established. The accuracy of this guidance system is particularly obvious in the highly vacuolate cells of the cambium, where the growing edge of the plate can be guided over a considerable distance to its final predetermined site (Figure 1.17D–F). 6. The new cross-wall must join and fuse with the mother cell wall.ref6 When the extending cell plate finally meets its appointed destination on the mother cell wall, further events must occur before the formation of two new daughter cells is completed. The membrane that surrounds the cell plate is topologically equivalent to the plasma membrane of the mother cell, and when these meet they fuse, initially at local sites, and later completely to effect the separation of two daughter protoplasts. At this stage, the new cross-wall, as it has now become, has a relatively homogeneous interior, and at this stage or shortly before, cellulose begins to be deposited. Since the cellulose is made at the plasma membrane (see Chapters 4 and 5), production of this structural polysaccharide takes place simultaneously at both faces of the new cross-wall. Cellulose fibers from one face do not intermingle with those from the other, and therefore, an “exclusion zone,” rich in pectic polysaccharides, is created in the center of the wall. This is called the middle lamella. At the junction between the mother cell and the new cross-wall, further events take place. In this region, local dissolution of the mother cell wall takes place, allowing the newly created middle lamella to extend and fuse with the middle lamella of the surrounding mother cell wall. It is as though the old wall were cut and the new walls were pasted in (Figure 1.18). The two new cross-walls, as they now are, become successfully integrated into the structure of the mother cell wall and finally create two daughter cells, each with its own new wall. The new three-way junctions of middle lamella that are created are important sites for future events (such as air space formation), which are discussed in Chapter 6 (Concept 6C5). 7. A plant is constructed from two compartments: the apoplast and the symplast.ref7 During the formation of new cross-walls, tubular elements of endoplasmic reticulum become trapped in the cell plate at the stage of vesicle fusion. The resulting channels are structurally elaborated into more permanent The Derivation of Cells and Their Walls anaphase chromosomes nucleus preprophase band of microtubules cell plate 11 preprophase band actinfree zone (A) actin (B) (C) actin (D) (E) (F) Figure 1.17 The role of the cytoskeleton in division plane alignment. (A) As a vacuolated dividing cell enters prophase, the preprophase band of microtubules at the cell cortex marks the site of the future division plane. Microtubules and actin also extend through the cytoplasm and connect with the nucleus (see Figure 1.15). (B) As the chromosomes align at metaphase, the microtubules in the preprophase band have disassembled, leaving in place an actin-free zone at the cortex throughout mitosis. (C) Actin persists elsewhere in the cell and helps guide the extending cell plate during cytokinesis to the correct site at the mother cell wall. On completion of cytokinesis, the cortical array of microtubules is reestablished. (D–F) Cutaway diagrams showing the division of an elongated and vacuolate cambium cell. These show the position of the preprophase band of microtubules (D), the pcw 1A5.2/1.17 extension of the cell plate following nuclear division (E), and the completed division (F) in which the cell plate has fused with the mother cell wall at the predicted site to create two new cambial cells. pores called plasmodesmata (singular, plasmodesma). Each pore is lined by plasma membrane that is continuous from cell to cell and usually contains a rodlike structure derived from the endoplasmic reticulum that is called the desmotubule. While many plasmodesmata are formed during cell plate formation, it is important to realize that they may also be formed de novo in more established cell walls. Although their diameter is on the order of 50 nm or so, the aqueous channel between the plasma membrane and the desmotubule that extends from cell to cell is rather tightly regulated and in general will allow the free passage of only small molecules. (Plasmodesmata are discussed in more detail in Concept 1C9.) The presence of abundant plasmodesmata means that all of the cells in a higher plant, with very few exceptions (stomatal guard cells and germ cells), share, topologically speaking, a single plasma membrane and thus the cells are cytoplasmically coupled to each other. This cytosolic continuity within the plant creates a single, extended topological space, bounded by shared plasma membrane, that is called the symplast (Figure 1.19). The remainder of the plant, which includes everything outside the symplast, also forms a continuous space, occupied by the cell walls, the intercellular spaces, and the contents of such dead cells as xylem vessels that have lost their plasma membrane. This continuum is called the apoplast (Figure 1.19) and is broadly equivalent to what we know as the extracellular matrix. cellulose microfibrils mother cell wall plasma new cross membrane wall (A) middle lamella (B) wall dissolution (C) new 3-way junction (D) Figure 1.18 Steps in completing a new cross wall and generating two new daughter cells. After the membrane around the cell plate has completely fused with the plasma membrane of the mother cell (A), cellulose begins to be deposited within the plate and a new middle lamella region is created in the central region (B). (C) Wall hydrolytic enzymes aid the controlled dissolution of a region of the mother cell wall that allows the cutting and pasting of the new wall into the old. (D) The middle lamellae become continuous, the two daughter cells acquire their own complete walls, and a new three-way junction is created. 12 Chapter 1 Cell Walls and Plant Anatomy Figure 1.19 Apoplast and symplast. Most but not all cells in the plant are connected to their neighbors by plasma membrane–lined cytoplasmic channels called plasmodesmata. These connections create two discrete topological compartments within the plant: the apoplast, consisting of everything outside the cell’s plasma membrane, including the dead xylem vessels and their contents; and the symplast, which consists of everything inside the plasma membrane—the plant’s collective cytoplasm. As shown in the inset, the cytoplasm of cell A is continuous with that of cell B via the (deliberately simplified) plasmodesma. cytoplasm (SYMPLAST) plasmodesmata cell wall (APOPLAST) cytoplasm of cell A (SYMPLAST) plasmodesma A cell wall plasma membrane B plasma membrane cytoplasm of cell B (SYMPLAST) xylem vessels (APOPLAST) Both apoplast and symplast can provide routes for the local transport of water and solutes. Water, ions,pcw and1A7.1/1.19 small signaling molecules, for example, may be transported through the apoplast. The same molecules, together with sugars, amino acids, and many other small organic molecules, may be transported from cell to cell within the symplast. The plasma membrane, which forms the boundary between apoplast and symplast throughout the plant, has an important function in regulating the transport of solutes between the two compartments. In some cases the movement of materials may be restricted to either the apoplast or the symplast. Symplastic restriction is exemplified by stomatal guard cells (see Concept 1C2). These become symplastically isolated from their neighbors early in their differentiation, when all of the plasmodesmata connecting them to adjacent cells are removed. Apoplastic restriction can be seen in the waterproof, suberinized wall layer, known as the casparian band, which acts as a barrier between the endodermal cells of the root to the movement of water and solutes through the wall (see Concept 1C4). The idea of apoplast and symplast helps focus our attention on the three-dimensional organized continuity of both cells and their extracellular matrix. B. Walls in Cell Growth and Differentiation 1. Cells become organized at an early stage into three major tissue systems.ref8 In the previous section we discussed how and where new cells and new walls arise. In this section we examine how cells grow and differentiate into a variety of cell types and how these cell types are assembled into the tissues, tissue systems, and organs of the mature plant. It is convenient, at this stage, to have a descriptive framework to make some sense of the complex variety of cell patterns we find in sections of different plant parts. A simple hierarchical system of anatomy is widely used in which plants are first broken down into organs (for example, root, stem, or leaf), each of which in turn is built from different arrangements of three tissue systems, the dermal system, the ground system, and the vascular system (Panel 1.1). The dermal tissue system comprises the outer covering of the plant, that is, the epidermis and its more complex replacements in older organs such as bark. The ground tissue system consists of supportive tissues (for example, parenchyma, collenchyma, and sclerenchyma, which help support and protect the vascular tissue system) together with storage