1comppd
advertisement
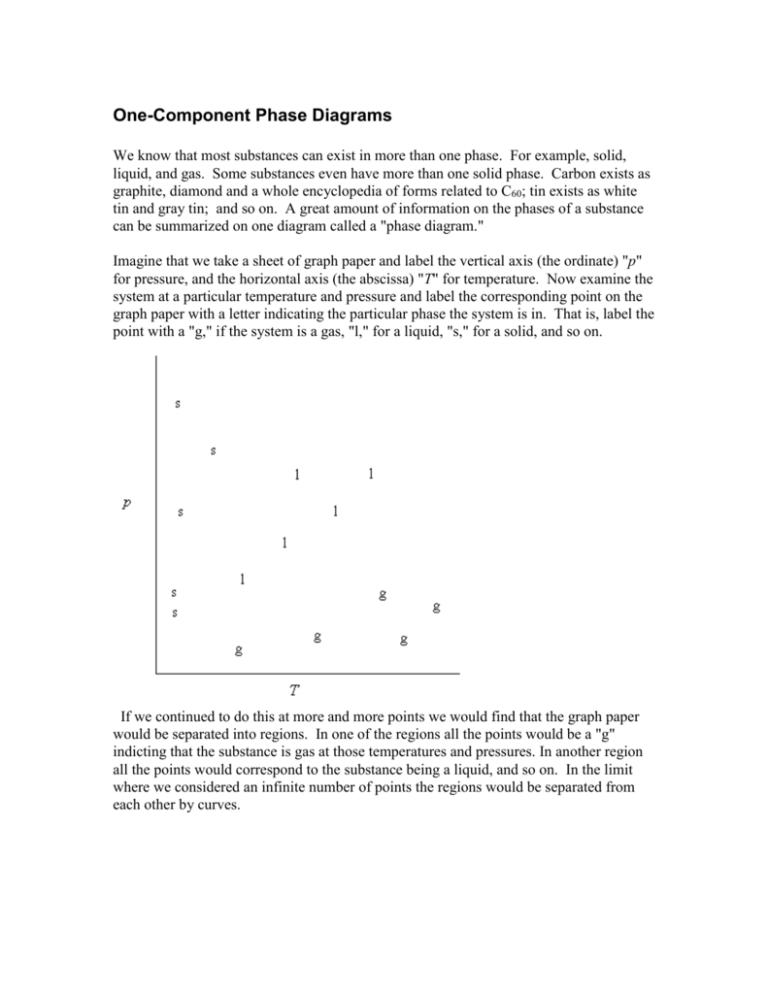
One-Component Phase Diagrams We know that most substances can exist in more than one phase. For example, solid, liquid, and gas. Some substances even have more than one solid phase. Carbon exists as graphite, diamond and a whole encyclopedia of forms related to C60; tin exists as white tin and gray tin; and so on. A great amount of information on the phases of a substance can be summarized on one diagram called a "phase diagram." Imagine that we take a sheet of graph paper and label the vertical axis (the ordinate) "p" for pressure, and the horizontal axis (the abscissa) "T" for temperature. Now examine the system at a particular temperature and pressure and label the corresponding point on the graph paper with a letter indicating the particular phase the system is in. That is, label the point with a "g," if the system is a gas, "l," for a liquid, "s," for a solid, and so on. If we continued to do this at more and more points we would find that the graph paper would be separated into regions. In one of the regions all the points would be a "g" indicting that the substance is gas at those temperatures and pressures. In another region all the points would correspond to the substance being a liquid, and so on. In the limit where we considered an infinite number of points the regions would be separated from each other by curves. Once we know the phase of a system in a particular region it is not usual to use so many g's and l's and s's. Most often we just label each region once. This diagram is an example of a phase diagram. The line separating the solid and liquid regions is called the "melting curve," the line separating the liquid and gas regions is called the "vaporization curve," or the "vapor pressure curve," and the line separating the solid and gas regions is called the "sublimation curve." In the regions between the lines only one phase, the phase indicated, can exist at equilibrium. On a line we can have two phases in equilibrium with each other. The point where the three curves meet is called the "triple point." At the triple point we can have three phases in equilibrium. (We will see later, after we derive the Gibbs phase rule, that a one component system can not have a quadruple point.) If the triple point has a lower pressure than one atmosphere the substance will have a normal melting point and a normal boiling point. The normal melting point is the temperature at which the substance melts at one atmosphere pressure. (As you can see the melting point will be slightly different at other temperatures,) The normal boiling point is the temperature where the substance boils at one atmosphere pressure. Another way to state this is that at the normal boiling point the vapor pressure of the substance equals one atmosphere. (You can also see from the above diagram that the boiling point of a substance varies quite strongly with changes in temperature.) As we learned in the early sections of the course, there is a point, called the critical point, "above" which the substance can not be converted to a liquid. That means that the vapor pressure curve must end at the critical point. At pressures above the critical pressures it usually does not make sense to try to distinguish between whether the substance is a solid or a liquid. In these cases the substances is referred to simply as a "fluid." CO2 is a substance that does not have a normal melting point or boiling point because the substance is not liquid at one atmosphere pressure. (Its triple point pressure is above one atmosphere.) A phase diagram for CO2 might look like the ones we have been showing, but the one-atmosphere line would be below the triple point. Solid CO2 is called "dry ice" because it never melts at pressures near one atmosphere. At one atmosphere pressure the solid "sublimes" directly from the solid into the gas phase. In a CO2 fire extinguisher you can hear the liquid "slosh around" inside the tank because liquid CO2 does exist at pressures above the triple point pressure. Water is an unusual substance because its melting point (273.15 K) is at a lower temperature than the triple point temperature (273.16 K). A schematic phase diagram for water might look something like, Note that the slope of the melting curve is negative. The slope of the melting curve is positive for most substances. The negative slope for water is an important feature and we will discuss it in more detail after we have derived the Clapeyron equation. For now we note that the negative slope of the melting curve for water probably is a key feature in the ability of our planet to support life. A completely labeled phase diagram for an ordinary substance might look like this, The dashed lines have been added simply to help locate the normal melting and boiling points of the substance and are not a necessary part of the phase diagram. WRS