Hund's rule -
advertisement

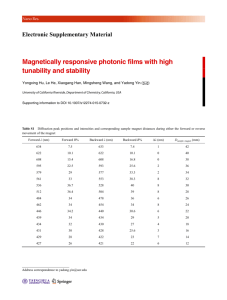
Hund’s Rule Investigation Chem. 319 Ms. Hobbie There are two important facets of Hund’s Rule: 1. Each orbital in an energy sublevel is occupied by a single electron before any orbital is doubly occupied. 2. All electrons in singly-occupied sublevels (unpaired electrons) will have spins in the same direction. Draw shorthand orbital notation diagrams (arrows) of the following elements, keeping Hund’s rule in mind. 25Mn 26Fe 27Co 28Ni 29Cu 30Zn Background Wolfgang Pauli discovered that electrons in an atom are spinning. Physics tells us that moving charges create magnetic fields. The arrows above represent electrons that are spinning; therefore… each electron represents a moving charge. each electron is a little magnet. Definitions to ponder What is the difference a paramagnetic substance and a diamagnetic substance? (P. 328 might be useful…) Create a hypothesis By looking at the orbital diagrams you have drawn above for these six elements, consider these questions: Which of these elements will be diamagnetic? Of the paramagnetic elements, which will have the greatest attraction towards a magnetic field? Experiment Record the mass of the compound containing the metal atoms of interest, both alone and when beneath a suspended magnet. Calculate the difference in mass and the percent of the sample’s mass that is reduced by the presence of the magnet Element DATA Mass w/o Mass with Magnet (g) Magnet (g) CALCULATIONS Difference % Difference in Mass (g) in Mass CLASS DATA Element % Difference in Mass 25Mn 26Fe 27Co 28Ni 29Cu 30Zn Analysis Explain how Hund’s rule is supported by the findings in this experiment.