Gummy bear lab
advertisement

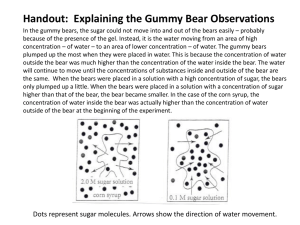
Name:____________________________________ Period:________________ Diffusion of Water with Gummy Bears (White) The purpose of this experiment is to observe the process of diffusion using a polymer. Gummy Bears are made of gelatin and sugar. Gelatin is a polymer that forms large threedimensional matrices, which give structural support to jellies, jams and lots of other things you use every day. Using what you know about osmosis and diffusion, write a hypothesis describing what you think will happen when a gummy bear is immersed for twenty-four hours in a weak sugar solution as compared to distilled water. After the experiments are done, we will answer the analysis questions using a jigsaw format. Hypothesis: If ____________________________________________________________________ (describe what will be done to the gummy bears) Then __________________________________________________________________ (describe what you think will happen) because ________________________________________________________________ (explain why you think this) Procedure 1. Obtain two beakers, four Gummy Bears in 2 different colors, two Post-Its, and two rulers. 2. On the top of each Post-It, write your names and class period. Then, label one Post-It "SUGAR WATER" and the other "DISTILLED WATER". 3. Place one Post-It on each beaker. 4. Measure each bear (in cm) from top to bottom (length) and from side to side (width) and from front to back (height). Record the centimeters in the data table. Use decimals. 5. Place the bears in the beakers and cover one with distilled and one with sugar water. 6. Place the cups on the counter. Let them sit overnight. 7. On the next lab day, record your observations without disturbing the bears. 8. Gently pour as much water out of the cups as possible without moving the bears. Then, slowly slide each Gummy Bear onto plastic wrap. Be very careful not to break the bears, they are very fragile. 9. Measure the length, width, and height. Record. Using a paper towel, blot the area around each bear dry. BE CAREFUL not to break the bears, they are very fragile. Name:____________________________________ Period:________________ Table 1: Quantitative Sugar Solution Observations Length (cm) Width (cm) Height (cm) Volume (cm3) Initial Size After sugar solution Qualitative Sugar Solution Observations Table 2: Quantitative Distilled Water Observations Length (cm) Width (cm) Height (cm) Volume (cm3) Initial Size After distilled water Qualitative Distilled Water Observations For the final piece of this activity you will be placing the bears you have already experimented on into a concentrated salt solution. First, write a hypothesis below about what you think will happen. Name:____________________________________ Period:________________ Next, CAREFULLY slide your bears into beakers of the prepared salt solution. Make qualitative observations during the next class. Was your hypothesis correct? Analysis We will work in expert groups to answer the analysis questions. You will answer your assigned question in your expert group, and then teach your group 1) the answer and 2) how you arrived at the answer (figured it out). Everyone will answer question 5. Use the back of this page to answer the questions in complete sentences. 1. Why are fresh fruits sprinkled with water at a market? 2. Roads are sometimes salted to melt ice. What does this salting do to the plants along the roadside? Why? 3. Why do fruits and beans swell when they are placed in water? 4. Why would a patient never have distilled water put directly into their blood stream? 5. Brea was interested in whether beetles will travel farther on light colored soil or dark colored soil. In this example, what is the independent variable and dependent variable? Name:____________________________________ Period:________________ Diffusion of Water with Gummy Bears (Purple) The purpose of this experiment is to observe the process of diffusion using a polymer. Gummy Bears are made of gelatin and sugar. Gelatin is a polymer that forms large threedimensional matrices, which give structural support to jellies, jams and lots of other things you use every day. Using what you know about osmosis and diffusion, write a hypothesis describing what you think will happen when a gummy bear is immersed for twenty-four hours in a weak sugar solution as compared to distilled water. After the experiments are done, we will answer the analysis questions using a jigsaw format. Hypothesis: (remember, If, Then, Because format) Procedure 1. Obtain two beakers, four Gummy Bears in 2 different colors, two Post-Its, and two rulers. 2. On the top of each Post-It, write your names and class period. Then, label one Post-It "SUGAR WATER" and the other "DISTILLED WATER". 3. Place one Post-It on each beaker. 4. Measure each bear (in cm) from top to bottom (length) and from side to side (width) and from front to back (height). Create a data table below in which to record your data. Please use decimals. 5. Place the bears in the beakers and cover one with distilled and one with sugar water. 6. Place the cups on the counter. Let them sit overnight. 7. On the next lab day, record your qualitative observations without disturbing the bears. 8. Gently pour as much water out of the cups as possible without moving the bears. Then, slowly slide each Gummy Bear onto plastic wrap. Be very careful not to break the bears, they are very fragile. 9. Measure the length, width, and height. Record. Using a paper towel, blot the area around each bear dry. BE CAREFUL not to break the bears, they are very fragile. Name:____________________________________ Period:________________ Table 1: Quantitative Sugar Solution Observations Qualitative Sugar Solution Observations Table 2: Quantitative Distilled Water Observations Qualitative Distilled Water Observations For the final piece of this activity you will be placing the bears you have already experimented on into a concentrated salt solution. First, write a hypothesis below about what you think will happen. Name:____________________________________ Period:________________ Next, CAREFULLY slide your bears into beakers of the prepared salt solution. Make qualitative observations during the next class. Was your hypothesis correct? Analysis We will work in expert groups to answer the analysis questions. You will answer your assigned question in your expert group, and then teach your group 1) the answer and 2) how you arrived at the answer (figured it out). Everyone will answer question 5. Use the back of this page to answer the questions in complete sentences. 1. Why are fresh fruits sprinkled with water at a market? 2. Roads are sometimes salted to melt ice. What does this salting do to the plants along the roadside? Why? 3. Why do fruits and beans swell when they are placed in water? 4. Why would a patient never have distilled water put directly into their blood stream? 5. Brea was interested in whether beetles will travel farther on light colored soil or dark colored soil. In this example, what is the independent variable and dependent variable?