Exam 3 Review Sheet HERE - SCF Faculty Site Homepage
advertisement

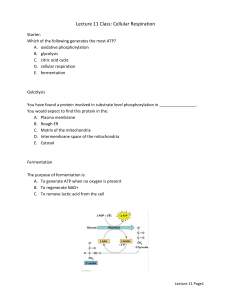
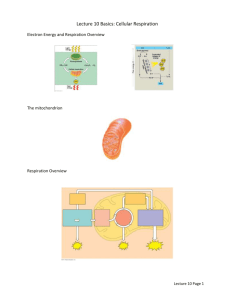
BSC 1007 REVIEW SHEET Chapters 6-8: Energy, Enzymes, ATP, Respiration, & Photosynthesis. Chapter 6 -What is Energy? -Differences between the Laws of Thermodynamics -What is Entropy? -Chemical Reactions -reactants -products -Exergonic vs Endergonic reactions -Coupled reactions -Reversible reactions -ATP (Adenosine Triphosphate) -Phosphorylation – (ADP + Pi ATP) -Substrate-Level phosphorylation -Chemiosmotic phosphorylation (electron transport phosphorylation) -Metabolic Pathways -Biosynthetic (building) -Degradative (breaking down) -What are Enzymes? -Characteristics of Enzymes. -catalysts (speed up reactions) -do not become part of the reaction -reusable -specific -temperature and pH sensitive -activation energy -Active Site -Regulation of enzyme activity -Inducers -Inhibitors -feedback inhibition Chapter 7 -Review oxidation & reduction -3 Stages of Aerobic Respiration -Glycolysis -where? -inputs & outputs -energy-requiring step -energy-releasing step -Krebs Cycle (Citric Acid Cycle) -where? -inputs & outputs -production of NADH, FADH2, CO2, & ATP -Electron Transport Chain -where? -inputs & outputs -production of ATP -final electron acceptor -Fermentation (“Anaerobic Respiration”) -Lactic Acid -Alcohol -know your 3 boxes! -know your numbers – NADH, FADH2, ATP Chapter 8 -review the nature of light (wavelenths/particles) -2 Stages of Photosynthesis -Light-Dependent Reactions -where? -inputs & outputs -chlorophyll -photosystems -electron transport -Light-Independent Reactions (Calvin Cycle) -where? -inputs & outputs -RuBP -Rearrangement steps -final (important) product! Extra Quiz: Energy and Life 1. Objects that are not actively moving but have the capacity to do so are said to possess: A. B. C. D. kinetic energy potential energy entropy living energy 2. The process by which an atom or molecule loses an electron is: A. B. C. D. reduction catalysis oxidation photosynthesis 3. Reactions that tend to go on their own, releasing energy, are called: A. B. C. D. endergonic exergonic catalytic productive 4. Enzymes are catalysts because they operate to: A. B. C. D. lower activation energy raise activation energy supply activation energy supply the reactants 5. The site on the surface of an enzyme where the reactant binds to the enzyme is called the: A. B. C. D. reactive site allosteric site active site binding site 6. The First Law of Thermodynamics states that _________________. 7. A measure of the degree of disorder in a system is called ___________. 8. In __________ reactions, the products contain more energy than the reactants. Answers: 1-B, 2-C, 3-B, 4-A, 5-C, 6-energy is neither created nor destroyed, 7-entropy, 8-endergonic