STATES OF MATTER TEST – STUDY GUIDE
advertisement

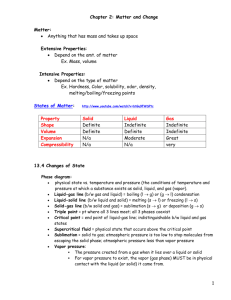
STATES OF MATTER TEST – STUDY GUIDE 1. Matter is anything that has mass and volume . Volume means the amount of space it takes up. 2. Properties of matter examples: color, taste, texture, odor, hardness, density 3. There are 3 main states or phases of matter on Earth: A. gas B. liquid C. solid A. Gas: very loosely packed molecules, no definite shape or volume B. Liquid: loosely packed molecules, definite volume, takes shape of container C. Solid: tightly packed molecules, definite shape and volume mass: how much there is of something; usually measured by weighing in grams on Earth property: a characteristic of matter; for example, shape, texture, size, color and what matter is made of. The properties of a playground ball are it is spherical, red, has a rough surface, is made of rubber, bounces, is hollow, and has a circumference of 15 inches. gram: a small metric unit used to measure mass; objects such as feathers, coins, and paper clips are measured in grams. kilogram: a thousand grams; people, large animals, boulders, cars, etc. atom: the smallest bit of matter Condensation: when water vapor is cooled and turned into a liquid Evaporation: when a liquid turns into a gas Temperature changes a solid into a liquid. Think of the sun melting an ice cube (solid) to water (liquid). Mixture: two or more things combined together Solution- a mixture with all parts evenly distributed Control: part of experiment that is not changed so scientists can use it for comparison Variable: part of the experiment that is purposely changed to find out what happens as a result Matter Study Guide KEY Chapter 5 1. How can I separate substances in a mixture? Separate matter by its physical properties 2. Imagine we have mixture of Wheat Chex, Rice Chex, Cheerios, pretzel sticks, marshmallows, and raisins but not all members of the group like each ingredient. What are the properties of each item and how will I separate them out? color, shape, texture, size, hardness 3. What is the effect of temperature on water in each of its states of matter? ice- heat above 0ºC melts to liquid water- heat above 100º C turns it to water vapor , below 00 C it freezes water vapor- temperatures below 100º C changes it to liquid 4. What are the differences between a chemical and physical change? Give an example of each. A chemical change means there is a new substance formed or a different type of matter than there was. a bruised apple A physical change means it is still the same type of matter even though it may have changed state, or shape or size or many other physical properties a chopped apple 5. Your Science project is coming up. What is the control versus the variable? What would happen if you did not have a control? The control is used to compare to the parts you are changing. The control is usually the ”normal” condition. The variables are the parts of the experiment you are changing or testing. 6. If I am buying drinks for our class and the store has various shapes and sizes, which properties will I be able to use to get the most for my money? size 7. Describe the relationship between matter and atoms. All matter is made of atoms. 8. If two items are on the scale, how will I know which items has more mass and which has less mass? The one with more mass pushes the scale down farther 9. Write an example of a physical change. cutting an apple, or paper or anything, changing the shape or size of a solid or liquid OR changing the state of matter to solid or liquid or gas 10. Write an example of a chemical change. baking cookies, apples rotting, rust, burning wood, ( smoke, ash, heat), cooking eggs Chemical energy includes any time a new substance is formed like smoke or gas, or energy is given off as in heat or light, or usually if there is a color change like when an apple starts to decompose or iron starts to rust.