Diffusion and Osmosis Labs
advertisement

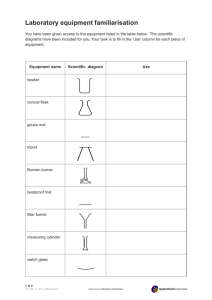
DIFFUSION THROUGH A MEMBRANE Background: The cell membrane acts as a barrier to some substances while allowing other substances to enter or leave a cell. The permeability of the cell membrane can change in response to changes in the cell’s external and internal environment. Some substances, such as water, oxygen and carbon dioxide, can pass freely through a cell membrane. Other substances have a more limited access to the cell. Homeostasis, the maintenance of the cell contents at a constant concentration, is dependent on the cell being able to regulate the substances that enter and leave the cell. In this activity a non-living cellophane membrane will be used as a model for a living cell membrane. Cellophane membranes are selective, but unlike living membranes, their selectivity does not vary. Water and other small molecules can pass through, but large molecules are blocked. Pre-Lab Questions: 1. Define diffusion and state two examples of diffusion in everyday life. 2. a) Define permeable, semi-permeable and impermeable. b) If an open window in your house is referred to as permeable, what would semi-permeable and impermeable be represented by? Procedure: 1. Soak a 10 cm length of dialysis tubing in water. Rub one end together between your fingers and open the tube completely. Tie one end tightly with a piece of string. 2. Place 5 mL of glucose solution and 5 mL of starch solution in the tubing. Tie the top of the tube tightly with another piece of string. Rinse the tubing under running water. 3. Place 200 mL of water into a beaker. Add 15 drops of Lugol’s iodine solution and mix well. Pour 2 cm of iodine/water solution into a test tube and place it into the beaker. Suspend the tubing from a ring clamp on a retort stand so that it is submerged in the beaker – try to prevent it from touching the sides of the beaker or the test tube. 4. Leave the beaker, tubing and test tube to sit overnight. 5. Construct a chart to record the observations. It should include the initial and final descriptions of the contents of the tubing, beaker and tube. 6. After leaving the equipment overnight, test the contents of beaker, test tube, and dialysis tubing for both starch and glucose. Record the results underneath your observation table. Post-Lab Questions: 1. Why did the tubing need to be rinsed under running water? 2. What sources of error could have been present in this experiment, or what precautions were taken to prevent error? 3. a) What is the positive test for starch? b) What is the positive test for glucose (a simple sugar)? 4. Was there any evidence of starch in the solution in the beaker? How could you tell? 5. What substances were present in the solution in the beaker? How could you tell? 6. What substances were present in the solution in the tubing? How could you tell? 7. Draw a diagram of the experimental setup, and summarize the movement of each substance in the experiment. 8. a) What is a starch molecule composed of? b) How does the structure of the starch explain the movement of this molecule in the experiment? c) Describe the structure of a glucose molecule. d) How does the structure of glucose explain the movement of the molecule in the experiment? 9. There were two types of movement illustrated in this experiment. What were they, and which substances experienced each type? OSMOSIS Osmosis is described as the movement of water from an area of high concentration to an area of low concentration without the use of energy. Solutions can be described as being isotonic, hypertonic or hypotonic based on the concentration of solvent and solute. Cells can become turgid or flaccid when placed in solutions that have different concentrations of water. Activity: 1. Use the potato coring tool to obtain four cylinders of potato, and cut each cylinder to a length of 3-5 cm. Record the length, diameter, and mass of each piece. Determine the volume of each piece of potato by the displacement of water method shown to the right. Make note of any qualitative observations as well (ex. its colour, whether it is turgid or flaccid, etc.). 2. Make up three salt water solutions. A bottle of 10% salt solution (sodium chloride) is provided. Label four beakers as follows: 0%, 1%, 5%, and 10% salt. You will need to make two of the salt water solutions by diluting the concentrated 10% stock solution to 5% and 1%. Use the following calculations to help you determine how much of the stock solution to use. a) concentration of stock solution I desired final concentration = amount of dilution b) 50 mL total volume Iamount of dilution (from a) = amount of stock (mL) c) 50 mL total volume – amount of stock (from b) = amount of water (mL) 3. Place one piece of potato in each beaker. Cover the beakers with plastic wrap or aluminum foil and an elastic band and leave them overnight. 4. Record the final length, diameter, mass, and volume of each potato segment and the final volume of the water in each of the three beakers. Also, make note of any quantitative changes in the potato. Observations: 0% 1% 5% 10% Change Ending Beginning Salt Concentration Mass Volume Diameter Length Qualitative Obs Mass Volume Diameter Length Qualitative Obs Mass Volume Diameter Length Post-Lab Questions: 1. What sources of error could have been present in this experiment, or what precautions were taken to avoid error? 2. Why was it important to cover each beaker? 3. a) Determine and record the difference in each potato segment’s length, diameter, mass, and volume. b) What happened overnight to the potato segments? Why? Address both quantitative and qualitative observations. 4. Identify each of your solutions as isotonic, hypertonic, or hypotonic and explain the identification. 5. Draw a diagram of the experimental setup and indicate the movement of substances in each beaker.