Chemistry Case Study Teacher's Notes
advertisement

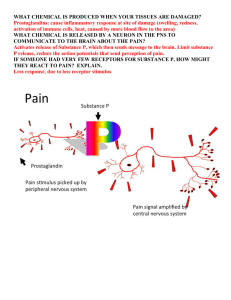
SCH 4U1 Case Study – Unit 2: Structure and Properties CASE STUDY: HOW EFFECTIVE IS YOUR PAIN RELIEF MEDICATION? ONTARIO CURRICULUM EXPECTATIONS Big Ideas: The nature of the attractive forces that exist between particles in a substance determines the properties and limits the uses of that substance. Overall Expectations: C1 Assess the benefits to society and evaluate the environment impact of products and technologies that apply principles related to the structure and properties of matter C2 Investigate the molecular shapes and physical properties of various types of matter; C3 Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. Specific Expectations: Relating Science to Technology, Society and the Environment C1.2 evaluate the benefits to society, and the impact on the environment, of specialized materials that have been created on the basis of scientific research into the structure of matter and chemical bonding Developing Skills of Inquiry and Communication C2.1 Use appropriate terminology related to structure and properties of matter, including, but not limited to: orbital, emission spectrum, energy level, photon, and dipole C2.3 Predict the shapes of simple molecules and ions, using the valence shell electron pair repulsion (VSEPR) model, and draw diagrams to represent their molecular shapes C2.4 Predict the polarity of various chemical compounds, based on their molecular shapes and the difference in the electronegativity values of the atoms. Understanding Basic Concepts C3.4 explain how the physical properties of a solid or liquid (e.g., solubility, boiling point, melting point, melting point suppression, hardness, electrical conductivity, surface tension) depend on the particles present and the types of intermolecular and intramolecular forces (e.g., covalent bonding, ionic bonding, Van der Waals forces, hydrogen bonding, metallic bonding) NOTES TO THE TEACHER: Rationale: This case study is meant to be used in the first unit of the Grade 12 UniversityPreparation Course. It is chemical, biological and somewhat sociological in nature. It is meant to embrace the theme of Science, Technology, Society and the Environment (STSE) and is motivated by the millions of people each day that use over-the-counter (OTC) pain-relief medication for aches and pains, as well as those whose physicians have prescribed certain pain-relief medications for more serious pains. Prior Knowledge: It would be beneficial for students to have already covered the organic chemistry unit of the Grade 12 University-Preparation Chemistry course, as some questions in the case study (particularly the drug information sheet) require knowledge of basic organic chemistry. However, the case study may still be given out, and students can research material with regards to functional groups. Regardless, a table of common functional groups from the organic chemistry unit has been included to aid in students’ understanding (Page 16 of this teacher’s notes and resources). Some inquiry questions consider concepts such as Valence Shell Electron Pair Repulsion Theory (VSPER), bonding, polarity and intermolecular forces which may have been covered in the Grade 11 Chemistry course and have been taught in great detail in the Grade 12 course. Requirements: Students need to provide a typed copy of their drug information sheet, the answers to the comprehension questions, and their report on a prescription pain medication. It is highly recommended that this case study be given out at the end of the Structure and Properties of Matter unit (Unit C). The due date should be such that it allows sufficient time for students to complete their research and analysis of their assigned drug (acetylsalicylic acid, acetaminophen or ibuprofen) and prescription drug. A one week period of time for students to work on this case study assignment is highly recommended. Students will work at home for the majority of that time. As per the preference of the teacher, students may be given one or two days to research in a library, computer lab or resource room. At the end of the research period, students will gather together in groups of three or four to discuss their findings and collectively determine which drug they think is the most effective. They will report to the teacher their issue, various perspectives, assessment criteria, and conclusions either at the end of the period or on the following day. Suggested Timeline: Day 1 – Hand out and explain various components of the case study assignment. Assign each student one of the three OTC medications. The teacher may assign expert groups allow students to choose their own expert groups. Each group should have at least one member representing each of the OTC medications. 2 Day 2-5 – Students will either have the opportunity to work on the case study assignment at home or on in-class research days that may be provided at the discretion of the teacher Day 6 and 7 – Students will gather together to discuss their research and findings and report them to the teacher. The group will record their discussions. Day 8 – All written work is to be submitted, and then assessed and evaluated. Modifications: When planning the time frame of this activity, please keep in mind the different cultures represented by the students in your class may have various religious holidays and so may not be in class attendance. Take this into consideration when giving them a due date for this case study assignment. This case study may be somewhat sensitive, as some students may know someone among friends or family that may have had some exposure to the overdose of both prescription and non-prescription medication. If a student does not feel comfortable doing this assignment, you may possibly suggest an alternate assignment. Identified/ELL Learners: Allow students who are identified learners to continue to use the resources that they have as per their individual education plan (IEP). Students in the ELL Program should also continue to use the resources provided to them in the school. These students may need extra time in the library or resource room. They may also require extra time to complete this assignment. Both identified/ELL students may need their assignments to be reprinted in larger text, while some may benefit from a scribe or translator respectively. Also, if these students need help, provide them with relevant resources and perhaps extra resources of tackling the problem of writing a report in APA format (including how to construct a works cited and using in-text citations within the report). Assessment and Evaluation: There are plenty of resources available for assessment and evaluation for this particular case study. In the student handouts is a copy of the marking scale for all three components of the assignment: the Comprehension Questions (Communication), the Drug Information Sheet (Thinking/Inquiry) and the Report on a Prescription Pain-Relief Medication (Application/Making Connections). This is the primary assessment of learning (summative assessment). The marking scale will be used to inform further instruction and commenting. To ensure that students are fulfilling the appropriate requirements of the Thinking/Inquiry and Application/Making Connection components, an assessment checklist has been provided as a student handout. Please keep in mind that the case study only fulfills three of the four categories of knowledge and skills. Knowledge and Understanding has been left out only because of its increased potential use in quizzes and tests. The remaining three categories have remained as they apply more to a case study of this nature. 3 One form of assessment that has been provided for teachers is the Teacher Assessment of Learning Skills (Appendix 1) to be used as assessment as learning (formative assessment). This can be used as a thorough breakdown for assessing both the written and oral components of the case study. The assessment has been modified to suit the new learning skills (Growing Success, 2010). A second form of assessment uses an achievement chart rubric for the written components of the case study assignment. This will assist in providing a more appropriate mark for each component, but is primarily an assessment as learning. (Appendix 2: Case Study Rubric – How Effective is your Pain Relief Medication?). On the final day of the case study when students in their expert groups report their findings to their teacher, the following assessment as learning can be used, where students will be assessed on their ability to define the issue, developing assessment criteria, group recording and organization and the justification of the expert group’s appropriate choice as to which over-the-counter pain relief medication is more effective. (Appendix 3: Decision-Making Rubric for extra groups). To ensure that all students in each expert group have put in a consistent effort into research, discussion and decision making, a learning skills rubric for peer assessment (Appendix 4: Peer Assessment of Group Members) has also been provided. This can be used for assessment as learning. Each student will have the opportunity to fill out the assessment for each of their expert group members. Resources for Students and Teachers: Primary Article for Comprehension Questions: Kimbrough, D. (2004). The Aspirin Effect: Pain Relief and More. ChemMatters. Retrieved from http://portal.acs.org/portal/fileFetch/C/CTP_005370/pdf/CTP_005370.pdf Noe, E. (2005). Finding Pain Relief Over the Counter. Retrieved from http://abcnews.go.com/Business/PainManagement/story?id=731159&page=1 CBC News. n.d. Over-the-counter pain relievers. Retrieved from http://www.cbc.ca/news/interactives/map-painrelievers Dearing, G. and American Pharmacist’s Association. (2008). Achieving Optimal Therapeutic Outcomes With Oral Over-the-Counter Analgesics: Assessing Benefit Versus Risk. Retrieved from http://clients2.picnet.net/aapa/images/stories/education_and_certification/PSC-OTCPA.pdf Drugs.com [Internet] or Drugs Information Online. (2000-2010). Prescription Drugs: Information, Interactions & Side Effects. Retrieved from http://www.drugs.com Harrison, K. (Update 2008). Chemistry, Structures & 3D Molecules. Retrieved from http://www.3dchem.com/index.asp 4 CASE STUDY COMPREHENSION QUESTIONS ANSWER KEY 1. “NSAI” stands for Non-steroidal Anti-inflammatory, and refers to drugs such as aspirin or ibuprofen. These drugs (and other NSAIs) can relieve pain, fever, and inflammation. 2. Prostaglandins are classified as lipid molecules. They are soluble in nonpolar solvents like oil, but are insoluble in polar solvents like water. They are made of carbon, hydrogen, and oxygen and have a five-membered ring, two carbon chains, and assorted double bonds and other groups. 3. The synthesis of all the different prostaglandins in the human body begins with arachidonic acid. Arachidonic acid is a long-chain fatty acid that is converted to the first prostaglandin, called PGG2, with the help of an enzyme called cyclooxygenase. PGG 2 is then converted to another prostaglandin called PGH2. From there various enzymes convert PGH2 into numerous other prostaglandins. 4. NASIs work by inhibiting the arachidonic pathway. They compete with the arachidonic acid for cyclooxygenase. They bind to the cyclooxygenase, preventing the arachidonic acid from doing so. Since arachidonic acid can no longer synthesize the first prostaglandin, PGG2, the entire process of prostaglandin synthesis is disrupted. 5. Acetaminophen works on an enzyme further down the arachidonic pathway. At this point the prostaglandin responsible for inflammation has already been synthesized, so acetaminophen cannot relieve inflammation. But since the prostaglandins responsible for fever and pain have not yet been synthesized, acetaminophen is still effective at relieving these symptoms. 5 DRUG INFORMATION SHEET ANSWER KEY Drug Scientific Name: Acetylsalicylic Acid Drug Brand Name(s): Aspirin, Ecotrin Using VSPER theory, predict and draw a three-dimensional structure of your drug. Use a solid line if the bond is in the plane of the page; a dashed line, if the bond is behind the page, and the wedged line, if the bond is out of the page. Choose three central atoms from your diagram above. Label them with the appropriate number. Then, identify the molecular geometry surrounding these three atoms by filling out the following table. Identify at least two functional groups in your drug molecule? How might this functional group play a role in the effectiveness of your drug? Benzene, carboxyl, ester, etc. 6 Is the molecule polar? How do you know? How might the polarity of the molecule effect the uptake of the drug? The molecule is polar. You can show it through the structure of the molecule. The carboxylic acid group is highly polar. Also, since like dissolves like and ASA dissolves in water, it must be polar. The drug is easy to take orally, because it is a polar molecule. List two pros for the use of this drug? - It slows down the formation of blood clots – a risk for people at risk of heart attack or stroke - Some studies show that it can be used to prevent various forms of cancer (CBC.com) List three side effects of the drug? Serious Side Effects: black, bloody, or tarry stools; coughing up blood or vomit that looks like coffee grounds; severe nausea, vomiting, or stomach pain; fever lasting longer than 3 days; swelling, or pain lasting longer than 10 days; or hearing problems, ringing in your ears. Less serious side effects may include: upset stomach, heartburn; drowsiness; or headache. (Drugs.com) Alternate Information Various Answers 7 DRUG INFORMATION SHEET ANSWER KEY Drug Scientific Name: Ibuprofen Drug Brand Name(s): Advil, Midol, Motrin, etc. Using VSPER theory, predict and draw a three-dimensional structure of your drug. Use a solid line if the bond is in the plane of the page; a dashed line, if the bond is behind the page, and the wedged line, if the bond is out of the page. Choose three central atoms from your diagram above. Label them with the appropriate number. Then, identify the molecular geometry surrounding these three atoms by filling out the following table. Identify at least two functional groups in your drug molecule? How might this functional group play a role in the effectiveness of your drug? Benzene, carboxyl, alkyl, etc. 8 Is the molecule polar? How do you know? How might the polarity of the molecule effect the uptake of the drug? The molecule is polar. You can show it through the structure of the molecule. The carboxylic acid group is highly polar. Also, since like dissolves like and ibuprofen dissolves in water, it must be polar. The drug is easy to take orally, because it is a polar molecule. List two pros for the use of the drug? - Relieves mild to moderate discomfort of headache, menstrual pain, pain from soft tissue injuries and following surgery - Relieves pain of broken bones, bruises and sprains in children (CBC.com) List three side effects of the drug? Serious Side Effects chest pain, weakness, shortness of breath, slurred speech, problems with vision or balance; black, bloody, or tarry stools, coughing up blood or vomit that looks like coffee grounds; swelling or rapid weight gain; urinating less than usual or not at all; nausea, stomach pain, low fever, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes); fever, sore throat, and headache with a severe blistering, peeling, and red skin rash; bruising, severe tingling, numbness, pain, muscle weakness; or severe headache, neck stiffness, chills, increased sensitivity to light, and/or seizure (convulsions). Less serious ibuprofen side effects may include: upset stomach, mild heartburn, diarrhea, constipation; bloating, gas; dizziness, headache, nervousness; skin itching or rash; blurred vision; or ringing in your ears. (Drugs.com) Alternate Information Various Answers 9 DRUG INFORMATION SHEET ANSWER KEY Drug Scientific Name: Acetaminophen Drug Brand Name(s): Tylenol, Tempra, etc. Using VSPER theory, predict and draw a three-dimensional structure of your drug. Use a solid line if the bond is in the plane of the page; a dashed line, if the bond is behind the page, and the wedged line, if the bond is out of the page. Choose three central atoms from your diagram above. Label them with the appropriate number. Then, identify the molecular geometry surrounding these three atoms by filling out the following table. Identify at least two functional groups in your drug molecule? How might this functional group play a role in the effectiveness of your drug? Benzene, amide, hydroxyl, etc. Is the molecule polar? How do you know? How might the polarity of the molecule effect the uptake of the drug? The molecule is polar. You can show it through the structure of the molecule. The carboxylic acid and amino groups are polar. Also, since like dissolves like and acetaminophen dissolves in water, it must be polar. The drug is easy to take orally, because it is a polar molecule. List two pros for the use of this drug? - It treats the pain of mild arthritis - It relieves the pain of osteoarthritis in the knees (CBC.com) List three side effects of the drug? The most common one is lightheadedness. Some people may experience trembling and pain in the side or the lower back. Other rare side effects include yellow skin or eyes, unusual bleeding or bruising, weakness, fatigue, bloody or black stools, bloody or cloudy urine, and a sudden decrease in the amount of urine. Overdoses of acetaminophen may cause nausea, vomiting, sweating, and exhaustion. Very large overdoses can cause liver damage. (Drugs.com) Alternate Information Various Answers TEACHER BACKGROUND INFORMATION FOR READING COMPREHENSION ARTICLE The Aspirin Effect: Pain Relief and More Correction to article: The structures of PGG2 and PGH2 on page 9 mistakenly had an extra bond added. The red arrows indicate where they were removed to correct the structure. The Discovery of Aspirin It is fairly common knowledge, at least among students of chemistry, that aspirin owes its origins to the plant world, specifically willow bark and meadowsweet. Although Edward Stone, an Englishman, is generally credited as being the first person to give a scientific description of the effects of willow bark (1763), the use of willow bark dates back as far as the 5th century BC. Hippocrates wrote about a bitter powder that could be extracted from willow bark and was useful to ease aches and pains and to combat fever. By the early 1800s it was known that the active ingredient was a substance called salicin. When salicin is ingested, the body converts it into salicylic acid. An Italian chemist by the name of Raffaele Piria was the first to synthesize salicylic acid from salicin. For many years salicylic acid and some close derivatives were administered in high does to combat pain, inflammation, and fever. But there were some serious drawbacks. Salicylic acid was extremely irritating to the stomach, in many cases actually causing serious bleeding in the digestive tract. They say necessity is the mother of invention, and the creation of modern aspirin provides a good example. A German man suffering terribly from arthritis required large doses of salicylic acid to control his pain. But the negative effects on his stomach made it difficult for him to take the necessary dosage. As luck would have it, his son, Felix Hoffman, was a chemist. He had the idea that the stomach irritation that salicylic acid was causing might simply be due to the fact that it was an acid. He reasoned that if he could convert salicylic acid into a related compound of lower acidity, perhaps the new compound might retain its pain and fever reducing properties but would no longer be as irritating. He replaced one of the phenol groups in the molecule with an acetate group--thus acetylsalicylic acid. Ironically, although Hoffman finished his studies of the effectiveness of acetylsalicylic acid by 1897, the product was not immediately considered to be of great value. But a couple of years later, Heinrich Dreser, one of the chief chemists at Bayer, recognized the significance and value of the product. He renamed it aspirin, and as they say, the rest is history. It represents one of the most successful products of its kind ever marketed. Amazingly, despite the widespread use of aspirin, little was known about how it actually worked. It wasn’t until the 1970s that a British scientist, John Vane, and his colleagues actually elucidated the mechanism of its operation. He and his fellow researchers, Sune K. Bergstrom and Bengt I. Samuelsson, were awarded the Nobel Prize in Medicine in 1982. The Discovery of Acetaminophen As mentioned in the article, acetaminophen was discovered by Harmon Northrop Morse in 1873, but did not find medicinal use until twenty years later. The Discovery of Ibuprofen Unlike aspirin, which was discovered near the end of the 19th century, and which was based on the properties of a compound already in use, ibuprofen was not discovered until 1969. Its discovery basically is an example of how many modern drugs are “discovered”—it was the result of a meticulous investigation of over 600 potential molecules. Some of them were similar to existing painkillers, such as aspirin, while others were originally designed to serve as weed killers. Some compounds showed initial promise, but eventually failed, either because their analgesic properties were not adequate or they had unacceptable side effects. Eventually ibuprofen emerged. Although it was not the most biologically active molecule tested, it had the least side effects. The Discovery of Naproxen Naproxen represents the most recent of the four most popular over-the-counter analgesics sold in the United States. It was developed by the Syntex drug company in 1976. Originally it was sold only via prescription under the name Naprosyn, and quickly rose to become the leading arthritis drug in the United States. According to an article in the Wall Street Journal, “Syntex reaped 80% gross profit margins that were the envy of the drug industry.” The patent expired in Dec. 1993, and within five weeks ten generic companies entered the marketplace. In 1994 the Food and Drug Administration (FDA) approved naproxen sodium as a nonprescription drug. A Drug That Did Not Make It In the early 1980s, the drug benoxaprofen came to market. It represented a modification of naproxen and showed great early promise. It had very powerful anti-inflammatory properties, and only required that one tablet be taken each day. During its first six weeks on the US market it was taken by approximately a halfmillion people. Unfortunately, serious side effects were soon noted. They included internal bleeding, kidney and liver damage, and sensitivity to sunlight. It was implicated in almost eighty deaths in the US and the UK. It was withdrawn from the market in 1982. Which Over-The-Counter Pain Reliever is the Best? There really is no simple answer to that question, and there can be specific reasons why a particular individual should avoid a particular medication. There are two basic choices—acetaminophen and the NSAIDs. These products are generally taken to relieve three kinds of symptoms: pain, fever, and inflammation. In general, acetaminophen can be a good choice when one is only seeking relief from pain and/or fever— a headache, or the aches, pains, and fever generally associated with the flu, for example. It is generally considered to be a very safe product when used according to instructions. It has the advantage of not causing stomach upset. Its major disadvantage is that it does not exhibit much anti-inflammatory activity. It can pose potential problems for individuals that have liver or kidney disease, so anyone for whom this might be a consideration should consult with their doctor before taking acetaminophen. It is commonly recommended that acetaminophen not be taken after the consumption of alcohol. Aspirin, at one time virtually the only choice for over-the-counter relief of pain, fever, and inflammation, has been replaced, to a large extent, by the other NSAIDs. It still functions well for the relief of all three symptoms, but potential side effects sometimes make it not the best choice, especially for some older patients. Aspirin differs from the other NSAIDs in that it can irreversibly alter the function of platelets in the blood that help in the clotting process. This property can be a disadvantage, but can also be advantageous, and has led to the common recommendation that older individuals at risk of heart attack and stroke take small doses of aspirin each day to “thin” their blood. Aspirin is no longer recommended for children because it is a potential cause of Reye’s Syndrome, a potentially fatal complication. Ibuprofen, sold under several different names such as Advil and Motrin, also do an excellent job of reducing pain, fever, and inflammation, and is considered to have fewer side effects. But products such as these are not free of risks. They can trigger digestive-system bleeding, especially in heavy alcohol users, and can cause kidney or liver damage, especially in older patients and individuals using diuretics. Naproxen and ketoprofen offer similar advantages and disadvantages. The standard recommendation to “consult your physician” is probably wise advice. More on Prostaglandins Prostaglandins were first isolated in 1935 by Ulf von Euler of Sweden. Since they had been isolated from human semen, it was assumed that they came from the prostate gland. Thus the name. Today we know that prostaglandins are found and are synthesized in virtually every cell in the human body, so the name is somewhat of a misnomer. The article explains that prostaglandins are made of three elements: carbon, hydrogen, and oxygen. They consist of a five-membered ring of carbon atoms with two chains attached containing a total of fifteen additional carbon atoms. They are synthesized from arachidonic acid. If you are interested in learning more about the structure of prostaglandins, there is a very good website that contains excellent structural representations. http://www.elmhurst.edu/~chm/vchembook/555prostagland.html The article presents some general information about how prostaglandins are synthesized within the body. As the article makes clear, the synthesis of all prostaglandins begins with arachidonic acid. With the help of the enzyme cyclooxygenase, arachidonic acid is converted to the first prostaglandin, labeled PGG2. This, in turn, is converted to another prostaglandin labeled PGH2. At this point, as the article states, “the party starts.” The more accepted jargon in science refers to this as the “arachidonic acid cascade.” PGH2 can be converted into several other prostaglandins. All the prostaglandins consist of an oxygenated cyclopentane or pentene ring with a heptenoic or heptanoic side and an octenol sidechain on adjacent carbon atoms of the ring. Letters such as “G,” or “H” in the designation of a prostaglandin are assigned according to what has been substituted on the cyclopentane/cyclopentene ring in the prostaglandin molecule and where they are located relative to the double bond, if one exists in the ring . The subscripted numbers like “1” or “2” indicate the number of double bonds in the sidechains. If you are interested in reading more about this entire process, which is quite complex, the following websites should prove useful. http://web6.duc.auburn.edu/~deruija/prostaglandins_2002.pdf http://www.cyberlipid.org/prost1/pros0001.htm http://holivo.pharmacy.uiowa.edu/46131/steroids/ch3/pg.html More about Cyclooxygenase There is still a lot of research going on in the area of cyclooxygenase and how it operates. There are at least three different enzymes involved. They have been labeled COX-1, COX-2, and COX-3, although strictly speaking COX-2 and COX-3 are not truly cyclooxygenases. They have been given these labels because they catalyze the next step in the cascade. Aspirin, ibuprofen, and naproxen work by inhibiting the COX-1 enzyme. As explained in the article, these medications compete with the arachidonic acid for the COX-1 enzyme. If COX-1 is busy interacting with these drugs, it is not available to trigger the formation of prostaglandins, so pain, fever, and inflammation are reduced. Unfortunately, as the article points out, these medications are not selective. They also reduce the formation of prostaglandins that carry out other useful biological functions such as protecting the stomach and intestines from irritation. Because acetaminophen acts further down the chain, it does not have the side effect of possibly causing stomach upset, but the other side of the coin is that it doesn’t reduce inflammation. There are now prescription drugs sold under names such as Celebrex and Vioxx that act on COX-2. This means that they can be taken for long periods of time without producing gastrointestinal problems, which is why they are often prescribed for arthritis sufferers. It is now thought that there is a COX-3 which occurs even further down the chain and may be what is inhibited by acetaminophen. Some Interesting Facts about Public Knowledge of NSAIDs Americans consume more than 50 billion non-prescription pain relief tablets every year. There has been a significant trend to allow former prescription drugs to be sold over-the-counter. Since the mid-1970s almost eighty prescription drugs have been given non-prescription status. A national survey of 1,000 adults found that 85% have taken a non-prescription reliever. Approximately one-third of adults report that they take one of these drugs weekly. But at the same time 47% say that they do not read the labels on the drugs they take. In addition, 85% did not know what is meant by the phrase “non-steroidal anti-inflammatory drugs.” They are generally not aware of the differences between aspirin, ibuprofen, acetaminophen, and naproxen. When asked to name a NSAID, more indicated a product that contained acetaminophen than aspirin even though acetaminophen is not a NSAID. Seventy-four percent did not realize that the use of aspirin was associated with stomach upset or bleeding. MAJOR FUNCTIONAL GROUPS