Khaled M
advertisement

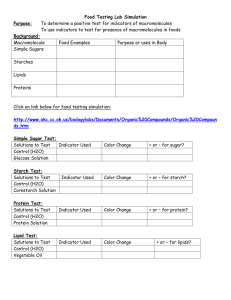
Khaled M. Mostafaa Mahmoud S. Morsyb a b King Abdul Aziz University, Institute for Research and Consultations, Jeddah, Saudi Arabia National Institute for Standards (NIS), Textile Department, El-Haram, Giza, Egypt Tailoring a New Sizing Agent via Structural Modification of Pregelled Starch Molecules Part 1: Carboxymethylation and Grafting Pregelled starch (PS) was subjected to acid hydrolysis using phosphoric acid to prepare pregeiied starches having different molecular sizes. The degraded pregeiied starches were carboxymethylated at different reaction times. The carboxymethyl derivatives were grafted with either methaeryiamide (MAam) or methaeryionitrile (MAN) as vinyl monomers using eerie ammonium nitrate (CAN) as initiator. Suitability of the new graft derivatives of pregeiied starch as sizing agent of cotton yarns was studied. It is shown by the data that the extent of carboxymethylation, expressed as carboxyl content, increases by increasing the extent of hydrolysis and reaction time. Furthermore, the graft yield, expressed as mmol MAam or MAN monomer/100 g graft copoly-mer (grafted carboxymethylated pregeiied starch or grafted carboxymethylated hydro-lyzed pregeiied starch) increases with increasing extent of carboxymethylation and degree of hydrolysis and follows the order: MAam > MAN. In addition cotton yarns sized with grafted carboxymethylated hydrolyzed pregeiied starch - irrespective of the grafting monomer used - have better mechanical properties (tensile strength, elongation at break, and abrasion resistance) than hydrolyzed pregeiied starches, carboxymethylated pregeiied starch and carboxymethylated hydrolyzed pregeiied starches. Keywords: Pregeiied starch; Carboxymethyl starch; Grafting; Methaeryiamide; Methaeryionitrile; Sizing; Mechanical properties 1 Introduction Although starch is widely used in the textile industry worldwide (about 75%) as a natural sizing agent for cotton fibers and cotton/synthetic fiber blends, it is not initially appropriate for sizing processes. This is due to its relatively high molecular weight and insolubility in water. Thus starch is often chemically modified to render it more suitable for such potential end use. Frequently used modification procedures are degradation, either by oxidation or hydrolysis [1-5], etherification [610], dextrinization [11], and grafting [12-22]. Of all these modifications graft copolymerization with vinyl monomers appears to be a very fascinating field for research with unlimited possibilities for improving starch properties. The present work was undertaken with a view of tailoring a new polymeric material based on pregeiied starch. For this purpose, the latter was first subjected to acid hydrolysis to vary the molecular size of the pregeiied starch. Pregeiied starches having different molecular sizes were then carboxymethylated (using monochloroacetic acid and sodium hydroxide) followed by grafting with methaeryiamide as hydrophilic monomer and methaeryionitrile as a hydrophobic one. In this way carboxymethyl groups as well as the synthetic vinyl polymer chains modify the molecular structure of pregeiied starch through controlling its molecular size and substituting some of the starch hydroxyl groups. Finally, utilization of the newly tailored polymeric pregeiied starch products in sizing of cotton yarns was tested to assess their suitability as sizing agents. 2 Experimental 2.1 Materials Pregelled starch was kindly supplied by Cairo Company for Starch and Glucose, Cairo, Egypt. Methaeryionitrile stabilized with 0.01 % hydroquinone was freshly distilled at 75°C and 13.33 kPa and stored at -10°C until used. Ceric ammonium nitrate was used in the form of 1 mol/ L solution prepared in 1 molar nitric acid. Methaeryiamide, monochloroacetic acid, sodium hydroxide, sodium carbonate phosphoric acid, dimethylformamide (DMF), and ethanol were pure grade chemicals (Aldrich, St. Louis, USA). Correspondence: KhaledM. Mostafa, King Abdul Aziz University, Institute for Research and Consultations, P. O. Box: 80271 Jeddah (21589), Saudi Arabia. E-mail: Khjnostafa@hotmail.com. 2.2 Preparation of hydrolyzed pregelied starch Three levels of hydrolyzed pregelied starches, namely H1- H2- H3-, starch having different degree of hydrolysis (expressed as copper number) were prepared using different concentrations (0.5, 1.0, 2 N) of phosphoric acid at 60°C for 0.5, 1.0 and 2 h using a starch to liquor ratio of 1:7.5. After the desired reaction time the reaction mixture was precipitated with 500 mL ethanol and neutralized with a dilute sodium carbonate solution, then washed and dried in an electric oven at 60°C for 3 h. The main characteristics of the pregelied starch and hydrolyzed pregelied starches are given in Tab. 1. Tab. 1. Main characteristics of pregelied starch and hydrolyzed pregelied starches as well as their statistical data. Substrate I: Pregelied starch. Substrate II-IV: Hydrolyzed pregelied starches. ± Values are the standard deviations of copper number measured three times for each sample. Detail of the conditions used is given in the experimental part. 2.3 Chemical modification 2.3.1 Preparation of carboxymethylated pregelied starch Pregelied starch before and after acid hydrolysis (3 g) was thoroughly mixed with 1.5 g monochioroacetic acid using an electric mixer, then freshly prepared catalyst (7.5 mL 4 N NaOH) was added. The reaction mixture was stirred vigorously and the reaction allowed to proceed at 60°C for 1 h in a thermostatic water bath. Subsequently, the mixture was poured in 500 mL of ethanol for precipitation, and the precipitate washed several times with an ethanol-water mixture (70:30) for 10 min each. It was found experimentally that three to five times washing with the latter mixture is enough to remove the contaminants (unreacted substances). This was evidenced by measuring the carboxyl content after washing for each sample till constant carboxyl content (three times measurements for each sample as well as their standard deviations) was obtained. Finally, the product was washed with pure ethanol and air-dried. 2.3.2 Polymerization procedure Graft polymerization was carried out in 100 mL-flasks containing an aqueous solution of grafting monomer (50% based on weight of substrate). The flasks were stoppered and placed in a thermostatic water-bath until the required temperature was reached. Nitrogen gas was purged through this solution to remove dissolved oxygen. The pregelied starch and eerie ammonium nitrate initiator were then added and the reaction mixture was mixed thoroughly. The content was shaken occasionally during polymerization. After the desired reaction time, the flask content was poured in 500 mL ethanol. At this end, a precipitate was formed which consisted of starch graft copolymer and homopolymer. In the case of poly(methacrylamide) the homopolymer was removed from the reaction mixture by washing several times with 400 mL ethanol-water mixture (70:30) (for 15 min each) at room temperature, the graft copolymer was filtered off and finally dried in an electric oven at 60°C for 2 h. It was found experimentally that washing five times with a 70:30 (v/v) ethanolwater mixture is sufficient for complete homopolymer removal in physical mixtures of starch/ poly(methacrylamide). This is evidenced by tracing the nitrogen content of the mixture after each wash until a constant value (± standard deviation) was obtained. Poly(methacrylonitrile) was removed from the reaction mixture by Soxhlet extraction using dimethylformamide (DMF) for 12 h at 30°C. It was found experimentally that, two times Soxhlet extraction with DMF for 12 h was sufficient for complete removal of poly(methacrylonitrile) from its mixture with the graft copolymer. Estimating the nitrogen content until constant value after each extraction until a constant value was obtained evidenced this. 2.4 Sizing of cotton yarns Cotton yarns 5 cm in width and 30 cm in length (kindly supplied by Misr Company for Spinning and weaving, El-Mehala El-Kobra) were padded through two dips and two nips in the cooked modified pregelied starch (10%) at 90°C to a wet pick-up of ca. 80% and dried in an electric oven at 100°C for 3 min. The sized yarns were finally kept at ambient conditions for at least 48 h before use. 2.5 Analysis Copper number was estimated by the micro-Briady method as modified by Heyes [23]. Carboxyl content was determined according to a reported method [24]. Nitrogen content was measured using the Kjeldahl meth- od [25] and apparent viscosity measured in a co-axial rotary viscometer (Haake RV20, Karlsruhe, Germany) at a shear rate of 516 cm-1 at 90°C. 2.6 Testing Tensile strength (TS) and elongation at break (%) were measured according to ASTM procedure D2256-66T. Abrasion resistance was determined using the Zweigle abrasion tester (Zweigle, Reutlingen, Germany). Both tensile strength, elongation at break and abrasion resistance were measured five times for each sample. 3 Results and Discussion 3.1 Carboxymethylation of pregeiied starch and hydrolyzed pregeiied starches During carboxymethylated of pregeiied starch derived from native pregeiied starch and hydrolyzed pregeiied starches in presence of sodium hydroxide the following main reaction is expected to occur: Fig. 1 shows the extent of carboxymethylation, expressed as mmol carboxyl groups/1OOg sample. The data show that the extent of carboxymethylation of pregeiied starch increases as the time of the carboxymethylation reaction increases. The same holds true for hydrolyzed pregeiied starches. It is also seen in Fig. 1 that the extent of carboxymethylation for hydrolyzed pregeiied starches is higher than that of pregeiied starch. The higher the extent of hydrolysis the higher the extent of carboxymethylation. An explanation is that acid hydrolysis of pregeiied starch increases the susceptibility of starch towards carboxymethylation. Acid hydrolysis decreases the molecular size of starch thereby increasing the surface area of starch without adversely affecting the starch hydroxyl groups, that are involved in the carboxymethylation reactions. 3.2 Combined effect of hydrolysis and carboxymethylation of pregeiied starch on grafting Figs. 2 and 3 show the effect of structural changes in the molecules of pregeiied starch, brought about by changing the amount of carboxymethylation (mmol COOH/100g sample), on the graft yield (mmol monomer/1 OOg sample) using methacrylamide and methacrylonitrile as monomers and eerie ammonium nitrate as initiator. The results (Figs. 2 and 3) signify the following main findings: 1- The graft yield obtained with hydrolyzed pregeiied starches is higher than that with native pregeiied starch; the higher the extent of hydrolysis the greater the graft yields. 2- The carboxymethylated pregeiied starches derived from hydrolyzed pregeiied starches exhibit much higher graft yields than the native and hydrolyzed pregeiied starches, which indicate that the combined effect of hydrolysis and carboxymethylation increases grafting. 3- The graft yield increases by increasing the carboxy-methyl content (mmol COOH/100g sample) of pregeiied starch and hydrolyzed pregeiied starches within the range studied. 4- The graft yields of the two monomers used onto carboxymethylated pregeiied starches follow the order: MAam > MAN. Fig. 1. Effect of duration of carboxymethylation of pregelled starch on the extent of carboxymethylation (expressed as mmol COOH/1OOg samples). ♦ = carboxymethylated pregeiied starch; ■ = carboxymethylated Hi-pregeiied starch); ▲ = carboxymethylated H2-pre-gelled starch); x = carboxymethylated H3-pregelled starch Reaction conditions: Pregelled starch, 3 g; monochloroacetic acid, 1.5 g; [NaOH], 4 N; solids to liquor ratio, 1:2.5; temperature, 60°C. Fig. 2. Graft yield (mmol metha-crylonitrile/1 OOg sample) of poly (MAN) - pregelied starch graft copolymer and poly (MAN) -carboxymethylated pregelied starch graft copolymers derived from native and hydrolyzed pregelied starches versus carboxymethylated content (mmol/ 100g sample). ♦ = carboxymethylated pregelied starch; ■ = carboxymethylated H1-pregelled starch); ▲ = carboxymethylated H2-pregelled starch); x = carboxymethylated H3-pregelled starch Reaction conditions: Pregelied starch, 3 g; [CAN], 0.006 mol/L; [MAN], 50% (based on weight of starch); soHds to liquor ratio, 1:7.5; time, 60 min; temperature, 45°C. Fig. 3. Graft yield (mmol metha-crylamide/1OOg sample) of poly (MAN) - pregelied starch graft copolymer and poly (MAam) -carboxymethylated pregelied starch graft copolymers derived from native and hydrolyzed pregelied starches versus carboxymethylated content (mmol/ 100 g sample). ♦ = carboxymethylated pregelied starch; ■ = carboxymethylated H1-pregelled starch); ▲ = carboxymethylated H2-pregelled starch); x = carboxymethylated H3-pregelled starch Reaction conditions: Pregelied starch, 3 g; [CAN], 0.006 mol/L; [MAam], 50% (based on weight of starch); solids to liquor ratio, 1:7.5; time, 60 min; temperature, 45°C. The enhancement in the graft-ability of pregelied starch by acid hydrolysis could be explained in terms of provision of larger surface area as shown before in carboxy-methylation. That is, the graft yield follows the order: grafted carboxymethylated H3-pregelled starch > grafted carboxymethylated H2-pregelled starch > grafted carboxymethylated H1-pregelled starch > grafted carboxymethylated pregelied starch. As stated before, when native and hydrolyzed pregelied starches were carboxymethylated before grafting, the susceptibility of the latter starch products towards grafting with the said vinyl monomers increases considerably. This reflects the role of carboxymethyl groups, which afford additional sites for grafting at the deprotonated carboxyl groups of carboxymethylated starch. The free radical is very likely formed at the oxygen atom of the carboxyl group. Furthermore, the presence of carboxymethylated groups along the starch chains opens up the structure of pregelied starch which facilitate diffusion of the monomers thereby leading to increased grafting. With this in mind, the following interaction scheme of the vinyl monomers with the carboxymethylated pregelied starches in the presence of initiator may be proposed: a- Initiation of grafting: b- Propagation of grafting: c- Termination of grafting: Where St-O-CH2-COOH is the carboxymethylated pre-gelled starch, R* is the free radical formed from the system and X represents functional group of the monomer, i.e. CONH2 and CN in case of methacrylamide and methacrylonitrile, respectively. The above postulation is in full agreement with previous reports dealing with grafting onto carboxymethylated cellulose [26-28]. On the other hand, the order of graftability of the monomers in questions could be associated with the difference between these monomers with respect to (a) polarizability of the vinyl double bond, (b) solubility of the monomers, (c) affinity of the monomer to pregelled starch and its ability to diffuse into the starch molecules and (d) ability of the monomer for homopolymerization. 3.3 Sizing of cotton yarns Cotton fibers are sized, i.e. coated with a strengthening adhesive-like material (usually starch or a starch-based material), to prevent damage during the weaving process. Size is usually applied to the warp thread, because this is particularly susceptible to mechanical strain during weaving. The work presented in this section is aimed at applying the newly tailored polymeric starch-derived products in sizing of cotton textiles and testing the suitability of these polymeric materials as sizing agent of cotton yarns. Tab. 2 shows important mechanical properties, i.e. tensile strength, elongation at break and abrasion resistance, of cotton yarns sized with native pregelled starch, hydro-lyzed pregelled starches, carboxymethylated pregelled starches and poly(MAam)-pregelled starch graft-copoly-mers derived from native and starches, hydrolyzed to different levels (H1-, H2-, and H3-starch), before and after carboxymethylation. Also, the physico-chemical characteristics of these products such as copper number; car-boxyl content and nitrogen contents are given in Tab. 2. 3.3.1 Tensile strength a- Tensile strengths of cotton yarns sized with native pregelled starch and hydrolyzed pregelled starches derived thereof amount to 230, 245, 240 and 225 g for native pregelled starch, H1-pregelled starch, H2-pregelled starch and H3-pregelled starch, respectively, as compared with a tensile strength of 210 g for unsized yarns. The values follow the order: H1-pre-gelled starch > H2pregelled starch > native pregelled starch > H3-pregelled starch. b- Tensile strengths of cotton yarns sized with carboxymethylated pregelled starches amount to 245, 260, 250 and 240 g for carboxymethylated native pregelled starch products, carboxymethylated H1pregelled starch, carboxymethylated H2-pregelled starch and carboxymethylated H3-pregelled starch, respectively. Tensile strength values follow the order: Carboxy- Tab. 2. Tensile strength, elongation at break and abrasion resistance as well as statistical data of cotton fabrics sized with different modified starch. - H1-, H2-, H3- P-St = pregelled starches hydrolyzed to different extents. - CM. n. P-St, CM. H1P-St, CM. H2-P-St, CM. H3-P-St = carboxymethylated native pregelled starch, carboxymethylated H-r, carboxymethylated H2-, and carboxymethylated H3-pregelled starches. - P (MAam)-G.n.P-St, P (MAam)-G.H1P-St, P (MAam)- G. H2-P- St, P (MAam)- G. H3-P-St = poly(MAam)-grafted native pregelled starch and pregelled starches hydrolyzed to different extents. - P (MAam) - G.CM. n. P-St, P (MAam) - G.CM. H1.P-St, P (MAam)-G.CM. H2.P-St, P (MAam)G.CM. H3.P-St = poly(MAam) - grafted carboxymethylated native pregelled starch and carboxymethylated pregelled starches hydrolyzed to different extents. ± Standard deviations of the above samples, which are taken as an average of five readings. methylated H1pregelled starch > Carboxymethylated H2-pregelled starch > Carboxymethylated native pregelled starch > Carboxymethylated H3-pregelled starch. c- Tensile strengths of cotton yarns sized with poly (MAam)-starch graft-copolymers amount to 255, 275, 265 and 245 g for grafted native pregelled starch, grafted H1-pregelled starch, grafted H2pregelled starch and grafted H3-pregelled starch, respectively. The values follow the order: grafted Hrpregelled starch > grafted H2-pregelled starch > grafted native pregelled starch > grafted H3pregelled starch. d- The tensile strengths of cotton yarns sized with poly (MAam)-grafted carboxymethylated starches amount to 260, 285, 275 and 250 g for grafted carboxymethylated pregelled starch, grafted carboxymethylated H1-pregelled starch, grafted carboxymethylated H2-pregelled starch and grafted carboxymethylated H3-pregelled starch, respectively. e- Finally, the increase in tensile strength of the sized cotton yarns follows the general order: grafted carboxymethylated pregelled starches > grafted pregelled starches > carboxymethylated pregelled starches > hydrolyzed pregelled starches > native pregelled starches. So, based on the obtained data, graft polymerization of methacrylamide with chemically modified pregelled starches greatly improves the sizability irrespective of the backbone of starch as evidenced by the data of Tab. 2. 3.3.2 Elongation at break Tab. 2 reveals that cotton yarns sized with the modified pregelled starches under investigation exhibit relatively higher elongation at break than the original unsized fabrics. Elongation at break follows the order: grafted car-boxymethylated pregelled starches > grafted pregelled starches > carboxymethylated pregelled starches > hy-drolyzed pregelled starches > native pregelled starches. 3.3.3 Abrasion resistance The results of Tab. 2 show that (a) the abrasion resistance of all sized yarns is higher than that of the unsized one, (b) the abrasion resistance of yarns sized with native pregelled starch and hydrolyzed pregelled starches (H1-, H2-, and H3- starches) amounts to 495, 534, 508 and 491 cycles against 480 cycles for unsized yarns, (c) the values of abrasion resistance of carboxymethylated pregelled starches amounts to 530, 570, 548 and 510 cycles for carboxymethylated pregelled starch, carboxymethylated H1 -pregelled starch, carboxymethylated H2-pregelled starch, and carboxymethylated H3-pregelled starch, respectively, as compared with 480 cycles for unsized yarns, (d) the abrasion resistance values of yarns sized with poly(MAam)-grafted pregelled starches amount to 548, 584, 562 and 525 cycles for grafted pregelled native starch, grafted H1-pregelled starch, grafted H2-pregelled starch, and grafted H3-pregelled starch, respectively. They follow the order: grafted H1-pregelled starch > grafted H2-pregelled starch > grafted pregelled starch > grafted H3-pregelled starch, (e) The abrasion resistance of yarns sized with poly(MAam)-grafted carboxymethylated pregelled starches exhibits the values of 558, 596, 575, and 538 cycles for grafted carboxymethylated pregelled starch, grafted carboxymethylated H1-pregelled starch, grafted carboxymethylated H2-pregelled starch, and grafted carboxymethylated H3-pregelled starch, respectively, as compared with 480 cycles for unsized yarns. Finally, as shown by the data given in Tab. 2, there is a direct relationship between tensile strength and abrasion resistance. 4 Conclusions - Of all hydrolyzed starches used, H1-starch is a suitable sizing agent. - All carboxymethylated starches under investigation are suitable sizing agents except that based on H3-starch. - All grafted starches serve as superior sizing agent. Or in other word, the combined effect of carboxymethyla-tion followed by grafting gives excellent results with respect to an improvement in mechanical properties of sized cotton yarns in comparison to that based on hydrolyzed pregelled starches, carboxymethylated pregelled starch and carboxymethylated hydrolyzed pregelled starches. Acknowledgement The authors wish to express their deepest thanks to H. Al-Bar, Professor of Organic Chemistry, Chemistry Department, King Abdul Aziz University, for providing all basic laboratory facilities as well as his interest and cooperation throughout the work. Also, great thank and appreciation goes to M. I. Khalil, Professor of Textile Chemistry, National Research Center, for his valuable discussions and analysis of different samples during this work. References [1] Y.-J. Wang, L. Wang: Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2003, 52(3), 207-217. [2] Y. Kato, R. Matsuo, A. Isogai: Oxidation process of water soluble starch TEMPO mediated system. Carbohydr. Polym. 2003,57(7), 69-75. [3] D. Kuakpetoon, Y.-J. Wang: Characterization of different starches oxidized by hypochlorite. Starch/Starke 2001, 53, 211-218. [4] A. Bayazeed, A. Higazy, A. Hebeish: Synthesis and application of reactive carbohydrates. Part VI: Application of reactive carbohydrates derived from starch and hydrolyzed starches to cotton fabrics. Starch/Starke 1989, 41,187. [5] Kh. M. Mostafa: Carboxyl containing starch and hydrolyzed starch derivatives as a size base material for cotton textiles. Polym. Degrad. Stab. 1997, 55(2), 125-130. [6] M. I. Khalil, S. Farag, Kh. M. Mostafa, A. Hebeish: Some studies on starch carbamate. Starch/Starke 1994, 46, 312-316. [7] U. Heinze, D. Klemm, E. Unger, F. Pieschel: New starch phosphate carbamides of high swelling ability: Synthesis and characterization. Starch/Starke 2003, 55, 55-60. [8] Z. Zhu: Starch mono-phosphorylation for enhancing the stability of starch/PVA blend pastes for warp sizing: Carbohydr. Polym. 2003, 54,115-118. [9] K. Haggag, F. A. Kantouch, I. Abd El-Talouth: Chemical modification of rice starch via its reaction with acrylamide. Angew. Makromol. Chem. 1989, 786(7), 169-179. [10] Kh. M, Mostafa: Evaluation of nitrogen containing starch and hydrolyzed starch derivatives as a size base material for cotton yarns. Carbohydr. Polym. 2003, 57, 63-68. [11] R. L. Whistler, J. N. BeMiller, E. F. Paschall: Starch Chemistry and Technology, 2nd Ed., Academic Press, Inc., Orlando, Florida, 1984. [12] M. I. Khalil, Kh. M. Mostafa, A. Hebeish: Synthesis of poly (methacrylic acid) starch graft copolymers using Mnlv-acid system. Starch/Starke, 1990, 42,107-111. [13] Kh. M. Mostafa: Graft polymerization of acrylic acid onto starch using potassium permanganate acid (redox system). J. Appl. Polym. Sci. 1995, 56, 263-269. [14] M. I. Khalil, Kh. M. Mostafa, A. Hebeish: Graft polymerization of acrylamide onto maize starch using potassium persuifate as initiator. Angew. Makromol. Chem. 1993, 273,14. [15] Kh. M. Mostafa: Graft polymerization of methacrylic acid on starch and hydrolyzed starches. Polym. Degrad. Stab. 1997, 55(2), 181-184. [16] Kh. M. Mostafa: Adding poly grafted starch to test properties of easy care cotton. Am. Dyestuff Rep. 1996, Sep., 85-87,91. [17] Kh. M. Mostafa: Synthesis of poly(acrylamide)-starch and hydrolyzed starches graft copolymers as a size base material for cotton textiles. Polym. Degrad. Stab. 1997, 55(2), 125-130. [18] Kh. M. Mostafa, A. A. El-sanabary: Utilizing polygrafted starch copolymers in easy-care finishing. Am. Dyestuff Rep. 1997, June 30-33. [19] Kh. M. Mostafa: Synthesis and characterization of poly (acrylonitrile) - pregeiied starch graft copolymers using eerie ammonium nitrate as initiator. Al-Azher Bull. Sci. 2001,12(1), 45-53. [20] Kh. M. Mostafa, N. S. Abdel Aziz: Preparation and characterization of poly (methacrylic acid) - cross-liked pregeiied starch graft copolymers as cation exchanger. Al-Azher Bull. Sci. 2001, 12(1), 55-64. [21] J. J. Meister, Mu Lan Sha: Synthesis, characterization, properties, and derivatives of poly (starch-g-(1 -amidoethylene)). I. Synthesis and characterization. J. Appl. Polym. Sci. 1987, 33(6), 1859-1871. [22] V. D. Athawale, V. Vidyagauri: Graft copolymerization onto starch. 3: Grafting of acrylamide using eerie ion initiator and preparation of its hydrogels. Starch/Starke 1998, 50(10), 426-431. [23] T. F. Hees, J. Sci. Chem. Ind. 1928, 47, T 90. [24] D. Daul, R.M. Reinhardt, J. E. Reid, Textile Res. J. 1953, 23, 719. [25] A. I. Vogel: Elementary Practical Organic Chemistry, Part 3, Quantitative Organic Analysis, 2nd ed., Longman Group Ltd., London, 1975, p. 652. [26] A. Kantouch, A. A. Hebeish, M. H. El-Rafie: Graft copolymerization of vinyl monomers on modified cottons. Part 1. Grafting of vinyl monomers on partially carboxymethylated cotton. Europ. Polym. J. 1970, 6, 1575. [27] F. E. Okieimen, D. E. Ogbeifun: Graft copolymerization of modified cellulose, grafting of acrylonitrile and methyl methacrylate on carboxymethylated cellulose. J. Appl. Polym. Sci. 1996, 59(6), 981-986. [28] M. L. Zhang, Y.-B. Tan, Z.-M. Li: Graft copolymerization of vinyl monomers on cellulosic materials. Angew. Makromol. Chem. 1998,260, 1,5-10. (Received: January 25, 2003) (Revised: September 20, 2003/December 30, 2003) (Accepted: January 5, 2004)