BRANDEIS UNIVERSITY
advertisement
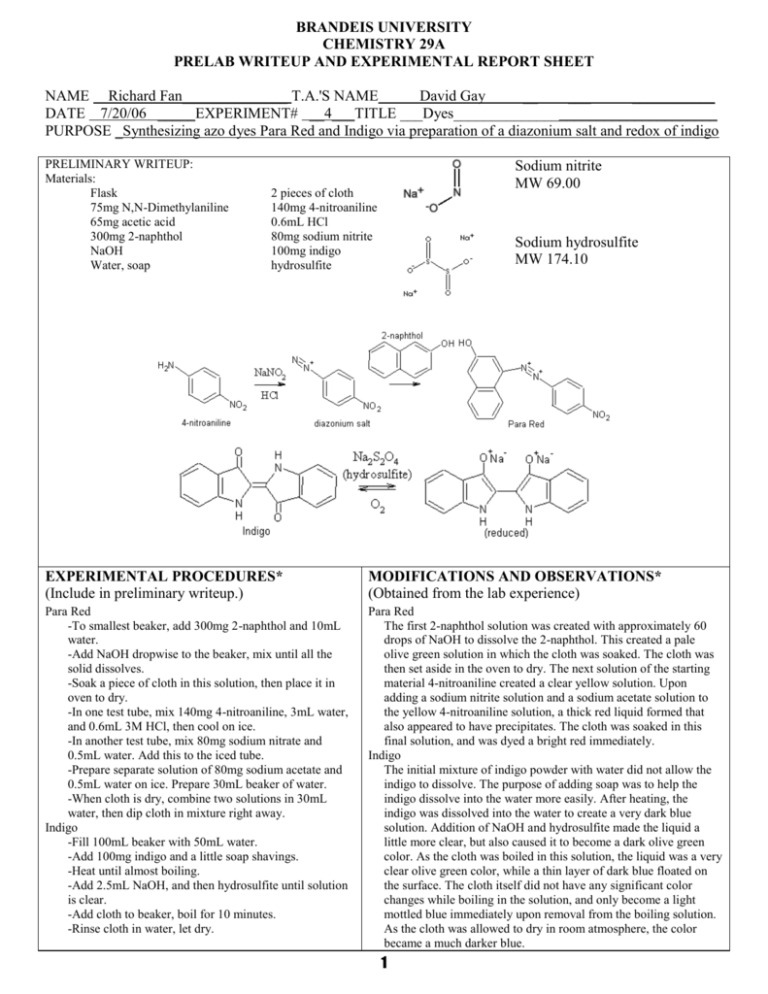
BRANDEIS UNIVERSITY CHEMISTRY 29A PRELAB WRITEUP AND EXPERIMENTAL REPORT SHEET NAME Richard Fan______________ T.A.'S NAME David Gay __ ___ ___________ DATE 7/20/06 _____EXPERIMENT# __4___TITLE ___Dyes___________________________________ PURPOSE _Synthesizing azo dyes Para Red and Indigo via preparation of a diazonium salt and redox of indigo PRELIMINARY WRITEUP: Materials: Flask 75mg N,N-Dimethylaniline 65mg acetic acid 300mg 2-naphthol NaOH Water, soap Sodium nitrite MW 69.00 2 pieces of cloth 140mg 4-nitroaniline 0.6mL HCl 80mg sodium nitrite 100mg indigo hydrosulfite Sodium hydrosulfite MW 174.10 EXPERIMENTAL PROCEDURES* (Include in preliminary writeup.) MODIFICATIONS AND OBSERVATIONS* (Obtained from the lab experience) Para Red -To smallest beaker, add 300mg 2-naphthol and 10mL water. -Add NaOH dropwise to the beaker, mix until all the solid dissolves. -Soak a piece of cloth in this solution, then place it in oven to dry. -In one test tube, mix 140mg 4-nitroaniline, 3mL water, and 0.6mL 3M HCl, then cool on ice. -In another test tube, mix 80mg sodium nitrate and 0.5mL water. Add this to the iced tube. -Prepare separate solution of 80mg sodium acetate and 0.5mL water on ice. Prepare 30mL beaker of water. -When cloth is dry, combine two solutions in 30mL water, then dip cloth in mixture right away. Indigo -Fill 100mL beaker with 50mL water. -Add 100mg indigo and a little soap shavings. -Heat until almost boiling. -Add 2.5mL NaOH, and then hydrosulfite until solution is clear. -Add cloth to beaker, boil for 10 minutes. -Rinse cloth in water, let dry. Para Red The first 2-naphthol solution was created with approximately 60 drops of NaOH to dissolve the 2-naphthol. This created a pale olive green solution in which the cloth was soaked. The cloth was then set aside in the oven to dry. The next solution of the starting material 4-nitroaniline created a clear yellow solution. Upon adding a sodium nitrite solution and a sodium acetate solution to the yellow 4-nitroaniline solution, a thick red liquid formed that also appeared to have precipitates. The cloth was soaked in this final solution, and was dyed a bright red immediately. Indigo The initial mixture of indigo powder with water did not allow the indigo to dissolve. The purpose of adding soap was to help the indigo dissolve into the water more easily. After heating, the indigo was dissolved into the water to create a very dark blue solution. Addition of NaOH and hydrosulfite made the liquid a little more clear, but also caused it to become a dark olive green color. As the cloth was boiled in this solution, the liquid was a very clear olive green color, while a thin layer of dark blue floated on the surface. The cloth itself did not have any significant color changes while boiling in the solution, and only become a light mottled blue immediately upon removal from the boiling solution. As the cloth was allowed to dry in room atmosphere, the color became a much darker blue. 1 RESULTS, DISCUSSION AND CONCLUSIONS* There are three separate steps for the preparation of the Para Red dye. The first step involves saturating a piece of cloth with a 2naphthol solution. Once dried, the cloth will have 2-naphthol on it which will react with the diazonium salt. The next step is the actual preparation of the diazonium salt. 4-nitroaniline reacts with sodium nitrate to produce 4-nitrobenzenediazonium chloride. When the cloth is soaked in this solution, the diazonium salt then diazotizes the compound already on the cloth and develops the dye on the cloth itself. The indigo dying process was a little different because the cloth is soaked with the dye solution and then the subsequent exposure to air causes the appearance of the dye color. The indigo powder is dissolved in water, but only with the help of soap because the soap is able to bond both polar and nonpolar compounds (water and indigo) in order to help the indigo dissolve more easily. The resulting light olive green colored liquid is the leuco form of indigo, which is a light yellow. The area where this leuco form meets the atmosphere (oxygen) is where the leucoindigo undergoes oxidation and reverts back to the original indigo form, causing the deep blue colored surface on the liquid. After the cloth was soaked in leucoindigo, it was removed and exposed to oxygen in the atmosphere where oxidation took place. As a result, the cloth which was not initially a very deep blue, progressively becomes a deeper color as the leucoindigo undergoes the oxidation reaction. The dying process of the cloth depends partially on the structure of the cotton material, which has a very large surface area on its fibers. This large surface area provides the area to which the dye can bond. Once bonded, it is difficult to remove the dye (ie: by washing with water) because the cotton fibers trap the dye molecules and make it hard for them to escape. In addition, indigo, for example, is not soluble in water in its native form. This also helps the dye remain on the cotton fiber. ANSWERS TO END-OF-CHAPTER QUESTIONS* 3. 4. - The first synthetic dye made was Mauveine, or aniline purple, and was discovered by William Perkin in 1856. The structure for the unknown ester X with formula C5H10O2 is most likely: O || CH3-CH2-CH2-C-O-CH3 The NMR shows that there are four hydrogen groups, two with two hydrogens each and two with three hydrogens each. This suggests that there are two CH2 groups and two CH3 groups. The main question is where the ester group is attached. The singlet peak (methyl group) at about 3.6ppm is most likely that attached to the primary carbon on an ester group. The remaining peaks, CH3, CH2, and CH2, must be attached to the secondary carbon on the ester group. 2