doc
advertisement

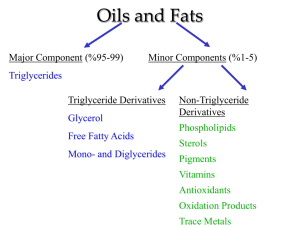
SCH4U Organic Chemistry Investigation 2: Saponification Saponification Since the esterification reaction is reversible it is possible to hydrolyze (from hydro, water, and lysis, to cut or break apart) an ester into a carboxylic acid and an alcohol. This reaction is also acid catalyzed. Esters can be broken apart under basic conditions, also. This reaction is called saponification because it can be used to make soap if one selects the appropriate ester (known as a triglyceride, usually obtained from animal fat) as starting material. The products of a saponification are an alcohol and the salt of a carboxylic acid as shown below. Pre-Lab: 1. Define Triglyceride: 2. What type of functional group is present in all triglycerides?___________________________________ 3. Define saponification: 4. Research and predict the products resulting from the reaction below. 5. Describe (with diagrams) the physical process by which soap solubilizes dirt. Materials: electronic balance; hot plate alcohol thermometer; stirring rod 10 mL graduated cylinder; 2 – 50 mL beakers 20 g coconut oil in a 50 mL beaker; 20 mL of 6.0 mol/L NaOH in 50 mL beaker piece of plastic wrap (10 cm x 10 cm) Safety Precautions: Wear safety goggles and a lab apron throughout the experiment. Do not allow the electrical cord from the hotplate to dangle over the edge of the bench. Listen carefully to the teacher’s instructions. If 6.0 mol/L NaOH solution gets on your skin, wash immediately with plenty of cold water. Notify the teacher. Be careful not to push the stirrer through the bottom of the Styrofoam cup when you are stirring. Procedure: 1. All observations must be recorded on a blank sheet of notebook paper with a detailed description identifying exactly what is being observed. 2. Label a small Styrofoam coffee cup with your initials. 3. At the same time, place the 20 g of coconut oil, in a 50 mL beaker, and the 50 mL beaker containing 20 mL of 6.0 mol/L NaOH using a hot plate. When these samples have reached ca. 45oC +/- 5oC, carefully pour both into the Styrofoam cup, with stirring. 4. Stir the mixture constantly for about 20 minutes until it has the consistency of cold honey. Take note of what you see. If the mixture does not thicken after fifteen minutes of stirring, let it sit for about three minutes. Then stir it for a few more minutes. 5. Lay some plastic wrap directly on top of the soap, in the Styrofoam cup. Leave the soap in the location suggested by the teacher. We will examine—and use—the hardened soap next class. Analysis Questions 1. What trends did you notice in the solubility of alcohols in water? Give explanations for why. Think about structures and what we have learned in class. [I] 2. Explain the difference in reactivity between sodium and short chain length alcohols like ethanol and longer chain alcohols like 1-pentanol. [I] 3. Write structural equations for each of the condensation reactions your performed. Provide the IUPAC name for all reactants and products. [A] 4. For each reaction describe the odour of the ester product. [C] 5. The ester methyl salicylate is also known as oil of wintergreen. Draw the structure methyl salicylate. Name some commercial products that you know contain this substance. [I][A] 6. Using a reaction sequence, explain how one of your esters may be converted to a soap. Include diagrams for all molecules. [I][A] 7. Give two errors that may have occurred during your experiment. [I]