Honors soap lab
advertisement

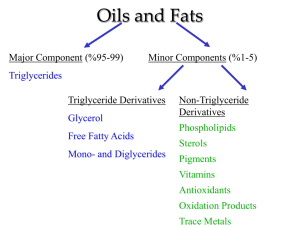
The Chemistry of Soapmaking Background The chemistry behind soap-making was not understood for many years. It is now known that saponification of soaps proceeds by the conversion of the triglycerides, which are the components of fats and oils, to fatty acid salts and glycerol as show in Figure 1. The R groups in the figure represent long carbon chains with the accompanying hydrogens. For each specific triglyceride, these specific R groups can be determined. For example tristearin gives the reaction shown in Figure 2. Prelab Write a purpose for the lab on your paper. (Think about the following questions when creating the purpose). Answer the questions in the prelab section of your lab using the two figures above and the textbook. 1. What reaction is described by the word saponification? What are the products from this reaction and what are the required reactants? 2. Draw out the full structure for three of the carboxylic acids listed in figure 1 and the image projected on the board. Using the textbook to help, identify all of the polar bonds, and 2. Find the polar and non-polar regions of the molecule. 3. What is the most dangerous part of the lab? Why is it critical to clean up all spills immediately? Safety issues: Hot sodium hydroxide is used in this lab so goggles must be worn at all times. Sodium hydroxide is very caustic so handle it with care. If you get some on your skin, wash it off immediately with water. Disposal: Check solutions to be discarded for excessive alkalinity (use the pH paper); if the pH is less than 10, the solutions may be poured down the sink; otherwise, they must go in the base waste container. The pH can be easily checked using pH paper aka Hydrion paper. Materials 3.16 g of lye crystals 9 ml of water 10 g Vegetable Oil (unsaturated) 15 g Crisco (saturated) Procedure Day 1: 1. While wearing safety goggles and neoprene gloves, combine solid lye and liquid in 150 ml beaker, stir well. Set aside and allow cooling (100° F to 125° F). This is best done in the hood with teacher guidance. WARNING: Always add your solid form lye, sodium hydroxide to the liquid. If the liquid were added to the solid form lye a violent reaction could result. 2. Place the Crisco in the small beaker labeled ‘oils’ and heat gently. Once the Crisco is liquid, remove from the hot plate and return to the front of the room to obtain the vegetable oil. Add the vegetable oil to the heated Crisco and stir. Adding the vegetable oil after the Crisco is heated will help cool down the oil. Once the fats and oils are melted allow the temperature to drop to 100° F to 125° F. Use the thermometer to determine the temperature. 3. Combine lye solution to the melted oils in the beaker labeled ‘oils’. Be careful not to splash while combining the mixtures. Stir (using the glass stir rod) until the mixture traces. If tracing takes more than 15 minutes, which it often does, stir for the first 15 minutes, then stir for 5 minutes at 15 minute intervals. Tracing looks like a slightly thickened custard, not instant pudding but a cooked custard. It will support a drop, or your stir marks for several seconds. Once tracing occurs... 4. Pour raw soap into your prepared molds. After a few days the soap can be turned out of the mold. If the soap is very soft, allow it to cure for a few days to firm the outside. 5. Cut soap into bars and set the bars out to cure and dry. This will allow the bar to firm and finish saponification. Place the bars on something that will allow them to breathe. Clean Up: Using soap, wash the two beakers and stirring rod. Make sure all traces of oil are absent from these tools before returning them to the red bin. Wipe and turn of the thermometer. The gloves are reusable; place them along with all other materials back in the red bin. Hard Water Reactions and pH: 1. Place about one-third spatula full of the soap you have prepared in a 50-mL beaker containing 25 mL of D.I. water. 2. Warm the beaker with its contents to dissolve the soap 3. Pour 5 mL of the soap solution into each of 5 test tubes (number them w/ tape so that you know which is which.) 4. Test no. 1 with 2 drops of a 5% solution of calcium chloride (5% CaCl2). Test no. 2 with 2 drops of a 5% solution of magnesium chloride (5% MgCl2). Test no. 3 with 2 drops of a 5% solution of iron (III) chloride (5% FeCl3) Test no. 4 with tap water. Test no. 5 with a piece of pH paper. What is the pH of your soap solution? 5. Record your observations on your lab paper. Post-Lab Questions 1. How can soap remove oil or dirt from clothes? 2. What was the role of the ethyl alcohol that was originally added to the reaction mixture?