Acidic Environment Notes (Student Produced)
advertisement

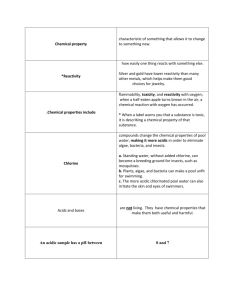
9.2 The Acidic Environment – Yr 12 2008 Written by Sam Jiang 1. Indicators were identified with the observation that the colour of some flowers depends on soil composition Classify common substances as acidic, basic or neutral Description Substance which in solution produces H+ or more strictly, H3O+ ( hydronium ion) Substances Acid Vinegar (acetic acid) Citric fruit (citric acid) Aspirin (acetyl salicylic acid) Vitamin C (ascorbic acid) Tartaric acid Car batteries (sulfuric acid) Base Substance which Bitter taste Caustic soda for oven/drain either contains the Soapy feel cleaners (NaOH) 2soluble oxide O or Conduct electricity in Washing soda (Na2CO3) Alkali hydroxide ion OH or solution (alkalis) Bicarbonate of soda (NaHCO3) which in solution Turn red limits blue Antacid (CaCO3, Mg(OH)2) which produces the Lime for mortar (Ca(OH)2) hydroxide ion Garden lime (CaCO3) + Neutral Substance which has same amount of H and OH- ions, e.g. Water Common properties Sour taste Sting or burn skin Conduct electricity Turn blue limits red Identify that indicators such as litmus, phenolphthalein, methyl orange and bromothymol blue can be used to determine the acidic or basic nature of a material over a range, and that the range is identified by change in indicator colour Indicators are chemical dyes that consist of organic molecules or ions with different coloured acidic and basic forms, they may change colour as the acidity of the surroundings changes. The range of acidity or alkalinity over which indicators change colour varies from one indicator to another. They generally have one colour when in Hln state and another colour when is ln-. When changing from one state to another, the indicator changes colour. Added to base: Hln + OH- H2O + lnAdded to acid: ln- + H3O+ Hln + H2O identify and describe some everyday uses of indicators including the testing of soil acidity/basicity Chemical Used by chemists in analytical work analysis Soil testing Monitoring and maintaining pools Monitoring waste e.g. detecting end point in titrations Soil is moistened Universal indicator is mixed with the sample Sprinkle white insoluble barium sulfate BaSO4 on the surface Match colour seen against PH/ colour chart Liquid ammonia NH3 and Agricultural lime CaCO3 increases basicity, Ammonium salts NH4+ increases acidity. Pool water’s acidity level need to be monitored Ensure skin/membranes are not irritated Avoid growth of green algal scam in pool Pool chlorine, Na2CO3, NaHCO3 and HCl, HNaSO4 are used 2. While we usually think of the air around us as neutral, the atmosphere naturally contains acidic oxides of carbon, nitrogen and sulfur. The concentrations of these acidic oxides have been increasing since the Industrial Revolution Identify oxides of non-metals which act as acids and describe the conditions under which they act as acids Oxides are a class of compounds that often display acidic or basic properties. Many non metal oxides are acidic oxide. Acidic oxide either reacts with water to form an acid or reacts with bases to form salts. Basic oxide reacts with acid to form salts. Common oxides Acidic Basic Amphoteric Neutral Valencies CO2 Na2O ZnO CO SO32- CO32SO2 K2 O Al2O3 NO NO3- NH4+ SO3 MgO PbO N2 O PO33- OHNO2 CaO SnO H3O+ SO42NO3 CuO GeO2 NH3 P 2 O5 Ag2O Sb2O3 P 2 O3 FeO Cl2O Fe2O3 Analyse the position of these non- metals in the Periodic Table and outline the relationship between position of elements in the Periodic Table and acidity/basicity of oxides Most metals and non metals can react with oxygen and produce oxide. Oxides of metals are basic, they are all ionic compounds. Oxides of non metals are acidic, they are covalent compounds Amphoteric oxides are formed by transition elements close to the non metals in the period table. Group 1 oxides are strongly basic, group 2 oxides are basic, basicity increases down the group. Most transition metal oxides are basic while some are amphiproteic Group 3-6, trend down group from acidic, amphoteric to basic oxides. Group 7 is strongly acidic, acidity decrease down the group Define Le Chatelier’s principle If the conditions of a system at equilibrium are changed, the system will readjust itself to oppose the change and re-establish equilibrium. If a closed system at equilibrium is disturbed, then the system adjusts itself so as to minimise the disturbance. Identify factors which can affect the equilibrium in a reversible reaction Temperature, increase will favour endothermic reaction, decrease will favour exothermic Volume, Increase favour the side with more moles of gas Pressure, increase favour the side with less moles of gas Concentration, favour to the side with less concentration Surface area for heterogeneous reaction Describe the solubility of carbon dioxide in water under various conditions as an equilibrium process and explain in terms of Le Chatelier’s principle CO2 + H2O H2CO3 Exothermic Pressure If the pressure increase, the equilibrium is disturbed, therefore the reaction tries to oppose this change by favouring the side with less number mole of gas, and the reaction will shift toward to the product side, more CO2 is dissolved, producing more H2CO3 and decrease the pressure to minimise the change. If the pressure decrease, the equilibrium is disturbed, therefore the reaction tries to oppose this change by favouring the side with more number mole of gas, and the reaction will shift toward to the reactant side, releasing more CO2 gases into the air and increase the pressure to minimise the change. Temperature If the temperature increase, the equilibrium is disturbed, since the reaction is exothermic, it will oppose this change by favouring the reverse reaction that absorbs heat, decreasing the temperature to minimise the change. Therefore the reaction will shift toward the reactant side. If the temperature decrease, the equilibrium is disturbed, since the reaction is exothermic, it will oppose this change by favouring the forward reaction that liberates heat, increasing the temperature to minimise the change. Therefore the reaction will shift toward the product side. Adding alkali CO2 + H2O H2CO3 H+ + HCO3- 2H+ + CO3- When a base is added, it will neutralise the H+ and form water, decreasing the concentration of H+. In order to re-establish equilibrium, more H2CO3 will move to the right to become H+, increasing the concentration of H+ , however this will decrease the concentration of H2CO3 and thus CO2 need to move to the right and form H2CO3. Analyse information from secondary sources to summarise the industrial origins of sulfur dioxide and oxides of nitrogen and evaluate reasons for concern about their release into the environment Identify natural and industrial sources of sulfur dioxide and oxides of nitrogen Explain the formation and effects of acid rain sulfur dioxide oxides of nitrogen industrial sources Power plant combusting fossil High temperature combustion in fuels or sulfur containing fuels motor vehicles (petrol engine), Combustion of fossil fuels in homes, industries and power plant. motor vehicles Metallic ore smelting facilities Wood and paper industrial processors Oil and gas refineries Natural sources Volcanoes Lightening Hot spring Combustion of organic matter, e.g. bushfire reasons for concern React with water in atmosphere and precipitate as acid rain. Harm or kill marine animals such as fish by increasing PH in water and creating a marine environment unsuitable for reproduction and survival. PH below 5 fish will stop laying eggs, lower PH will kill them, affecting all animals in the food chain. Reduces crop yields both by damaging foliage and by causing the leaching of minerals and important nutrients needed for plant growth from the soil and releasing toxic heavy metals. It is causing the defoliation of pine plantations, which is environmentally detrimental and destroying large amount of forest. Buildings made of steel are damaged by acid rain, steel bridge and building needs to be painted to prevent this. Building, statues stones and Lung damage, other respiratory monuments made by marble are illness particularly for asthma damaged by acid rain, forming patients. gypsum Fatigue, nausea, cough and CaCO3(s) + H2SO4(aq) vomiting. Lower resistance to CaSO4(aq) + CO2(g) + H2O(l) respiratory infections such as Calcium sulfate will flakes off, influenza. damaging the building. Photochemical smog Difficulty in breathing, choking sensation. Chronic lung, heart However the major source of oxides of nitrogen and sulphur dioxide is from burning fossil fuels for industrial operations and travelling both on the road and on air. These operations have developed society enormously, and created huge impacts on transport and living standards. Thus the negative effects of emissions must be balanced with the benefit received. Alternative energy sources are researched at the moment and methods to reduce the level of acidic oxides in the environment Describe, using equations, examples of chemical reactions which release sulfur dioxide and chemical reactions which release oxides of nitrogen 2ZnS(s) + 3O2(g) 2ZnO(s) + 2SO2(g) (burning sulfur compound) S(s) + O2(g) SO2(g) H2O(l)+ SO2(g) H2SO3(aq) 2SO2(g) + O2(g) 2SO3(g) H2O(l)+ SO3(g) H2SO4(aq) N2(g) + O2(g) 2NO(g) (lightening) 2NO(g) + O2(g) 2NO2(g) H2O(l) + 2NO2(g) HNO2(aq) + HNO3(aq) Assess the evidence which indicates increases in atmospheric concentration of oxides of sulfur and nitrogen “Increased” erosion of stone, marble monuments and buildings. Average concentration of SO2 and NO2 in most large cities worldwide is about 0.01ppm for each gas. This compares with measurements of 0.001ppm in other populated parts of the earth and as low as 0.00005 ppm in areas well away from human activity, electrical storms and volcanic action. This implies that concentration has increased. 3. Acids occur in many foods, drinks and even within our stomachs Define acids as proton donors and describe the ionisation of acids in water Acids are proton donors because in solution, they give up a hydrogen in or hydronium ion to the solution. Most hydrogen atoms contain no neutrons, but only a single proton in their nucleus. In explaining acidity, the terms “hydrogen ion” and “proton” are used interchangeably. Acids ionise in water to produce hydronium ion and a negative ion. Identify acids including acetic (ethanoic), citric (2-hydroxypropane-1,2,3- tricarboxylic), hydrochloric and sulfuric acid acetic (ethanoic), CH3COOH citric (2-hydroxypropane-1,2,3- tricarboxylic), C6O7H8 hydrochloric, HCl sulfuric acid, H2SO4 Describe the use of the pH scale in comparing acids and bases PH is an expression for the concentration of H+ ions in solution. However, PH measures the free hydrogen ion concentration in solution, not the total ions in solution. Neutral substances have pH around 7, acid is below 7, acidity increase as pH decrease, base is above 7, basicity increase as pH increase. Describe acids and their solutions with the appropriate use of the terms strong, weak, concentrated and dilute Describe the difference between a strong and a weak acid in terms of an equilibrium between the intact molecule and its ions Strong acids are those in which all the acid molecule present in solution has ionised to hydrogen ions, it has a high degree of ionisation and ionise completely, containing no intact molecules. The reactions are non reversible go to completion. HCl, HI, HBr, HNO3, H2SO4 NaOH, KOH, Ba(OH)2, Ca(OH)2, Na2O Weak acid are those in which only some of the acid molecules present in the solution has ionised to form hydrogen ions. There is an equilibrium holds between intact molecule and hydrogen ions as the reaction does not go to completion. Degree of ionisation increases for weak acid as they are diluted. CH3COOH, C6O7H8, HF, HNO2, H3PO4 NH3, CO32-, Concentrated solution means the solution is really concentrated, high number of moles of the acid is dissolved in an unit volume. Dilute solution means the solution has a low concentration, low number of moles of acid is dissolved in an unit volume. Identify pH as -log10 [H+] and explain that a change in pH of 1 means a ten-fold change in [H+] PH = -log10 [H+] [H+] = 10-PH 14 PH = 14 + log10 [OH-] = -log10 10 [OH ] Water partially ionises to produce the following equilibrium H2O H+(aq) + OH-(aq) [self ionisation equilibrium] The ionisation constant of water KW = [H+][OH-] =1.0 10-14 at 25 degree Gather and process information from secondary sources to explain the use of acids as food additives A diverse range of acids are used as food additives for various purposes: Acetic acid is the major component of vinegar. It is used for pH reduction, controlling microbial growth (fungi, moulds, yeasts, etc.) and flavouring. Citric acid is used for flavouring, microbial growth and pH reduction, also used for artificial lemon juice, citric flavoured drinks. Prevent fruit from browning by inactivates the enzymes that cause discolour. Malic acid imparts a smooth, tart taste and has an acid flavour, helping to mask the aftertastes of low or non-caloric sweeteners. It is primarily used in carbonated beverages, powdered juice drinks, jams and jellies, canned fruits and vegetables and confectionery. Phosphoric acid is primary used in cola, root beer and similarly flavoured carbonated beverages. Tartaric acid occurs in grapes and wine. It contributes a strong tart taste, which enhances fruit flavours, particularly grape and lime. It is often used as an acidulant in grape, wine and lime-flavoured beverages, gelatine desserts, jam and jellies and hard sour confectionery. As food additives, acids have the following main uses: Flavouring: each acid has a set of taste characteristics. Generally, weak acids have a stronger taste than strong acids because they exist in the primarily un-dissociated state. PH regulation: lowering the pH of the food system enhances the effectiveness of food preservatives. PH also determines the flavour and the microbial stability. Buffering: this is important because a fluctuating pH can have an adverse effect on flavour, colour and microbial stability. Common buffers: citric acid-sodium citrate. Preservation: acids help slow down the growth of spoilage organisms, especially bacteria. This effect very much depends on the pH value of the acid. Yeasts and moulds grow within a large pH range, and the pH must be lowered to halt their growth. Compare the relative strengths of equal concentrations of citric, acetic and hydrochloric acids and explain in terms of the degree of ionization of their molecules Hydrochloric acid is stronger than citric acid, which is stronger than acetic acid. HCl is a strong acid that completely ionises in solution, and thus it is stronger than citric and ethanoic acid, which are weak acids. Citric acid is a triprotic acid, which contains three replaceable hydrogen ions, and this ionises to a greater extent than ethanoic acid when placed in solution. Thus, citric acid is stronger than acetic acid. Identify data, gather and process information from secondary sources to identify examples of naturally occurring acids and bases and their chemical composition HCl - produced by glands in the lining of our stomachs to form an acidic environment for the efficient operation of the enzymes that break complex food molecules into easily transportable small molecules that are absorbed into the blood stream when they pass into the intestine. Acetic acid (CH3COOH) – is present in vinegar(4%)which is commonly made from wine by oxidation of ethanol, find naturally in apple, coca, grape, etc. Clear, colourless, distinctive odour, dissolve in water. Citric acid (2-hydroxypropane – 1,2,3 – tricarboxylic acid C6H8O7 ) – occurs in citrus fruit such as organs, lemons and lime. It is soluble white powder. Ascorbic acid (C6O8O6) – vitamin C. it occurs widely in fruits and vegetables and is an essential part of our diet. Used as an antioxidant in food preservation. Carbonic acid (H2CO3) – formed when carbon dioxide dissolves in water. Present in soda water Formic acid (HCO2H) – present in the stings of ants, bees and nettles Lactic acid (CH3CH(OH)CO2H) – present in milk and yoghurt. Produced by muscles during exercise. Bases Caffeine – C8H10N4O2 it is white crystalline powder with bitter taste found in coffee, colanuts, guarana, tea. Nicotine - C8H14N4 it is liquid obtained from tobacco leaves. Quinine (CH3NH2 – natural base found in rotting fish Ammonia (NH3) - Component of decaying organic matter, volcanic gases. It is colourless with sharp odour. Very few soluble bases exist freely in nature. However, calcium carbonate magnesium carbonate are found in rocks such as limestone, marble and dolomite. 4. Because of the importance of acids, they have been used and prevalence and studied for hundreds of years. Over time, the definitions of acid and base have been refined Outline the historical development of ideas about acids including those of: -Lavoisier Antoine Lavoisier, in about 1779 proposed that acids were substances than contained oxygen since he discovered that oxygen exists in many compound that displayed acidic properties when dissolved in water. However this theory was soon disproved because many oxygen containing substances were basic and some acidic substances were acidic but contained no oxygen. He focused on composition rather than property of acids. The presence of oxygen in compounds formed from non-metals causes acidity. -Davy Humphrey Davy, in 1810 suggested that acids were substances that contained hydrogen. Bases were substances that reacted with acids to form salts and water. This redefined acids in terms of the element hydrogen. These definitions worked quite well for most of that century, yet this is still a definition by composition and did not explain why acids/bases behave the way they do. All acids contain hydrogen. -Arrhenius Svante Arrhenius in 1887 proposed that an acid was a substance which ionised in solution to produce hydrogen ions and a base was a substance that in solution produced hydroxide ions. This introduced the neutralisation equation and he proposed that it was these ions that gave acids their characteristics properties. However this definition applies only to aqueous solutions and excludes substances that are distinctly basic such as NH3 NaCO3 with no OHAn acid is a substance that provides H+ ions in aqueous solution A base us a substance that provides OH- ions in aqueous solution Gather and process information from secondary sources to trace developments in understanding and describing acid/base reactions Outline the Brönsted-Lowry theory of acids and bases There are some inadequacies with Arrhenius definition of an acid, because it does not give due recognition to the role of the solvent. Ionisation of the acid is not something the acid does in isolation: rather it is a reaction between the acid molecule and the solvent. Whether an acid is strong or weak depends not only upon the nature of the acid itself, but also upon the nature of the solvent it is dissolved in: HCl in water is a strong acid, but when dissolved in diethyl ether it is quite weak. Also, some compounds that do not contain hydroxide ions are able to neutralise acids, such as ammonia, some salts can act as acids or bases and some substances can act as both an acid and a base. In 1923 two chemists. Lowry from Britain and Bronsted from Denmark, independently proposed new definitions for acids and bases in terms of proton donors and acceptors: these definitions overcame the difficulties regardless of solvent, and are the most widely used definition today, as it explains acid/base by property and behaviour rather than composition. An acid-base reaction is one in which a proton is transferred from an acid to base. Describe the relationship between an acid and its conjugate base and a base and its conjugate acid Identify conjugate acid/base pairs A base after it has received a proton has the potential to react as an acid, this is called conjugate acid. Similarly an acid which has donated a proton is potentially a base, called conjugate base. Acid Base Strongest Acid HCl Cl Weakest Base H2SO4 HSO4 HNO3 NO3H 3 O+ H2 O HSO4 SO42H3PO4 H2PO4CH3COOH CH3COOH2CO3 HCO3H2 S HSNH4+ NH3 H2 O OHHSS2Weakest Acid OHO2Strongest Base Strong acid has a weak conjugate base Strong base has a weak conjugate acid Weak acid has a strong conjugate base Weak base has a strong conjugate acid Identify a range of salts which form acidic, basic or neutral solutions and explain their acidic, neutral or bases basic nature Salt can be acidic, basic or neutral. Acidic and basic salts contain an ion which behaves as an acid or base. Ammonium chloride: acidic, combination of a strong acid and a weak base. HCl + NH3 NH4Cl Ammonium nitrate: acidic, combination of a weak base and a strong acid HNO3 + NH3 H3O+ + NO3- + NH3 Sodium carbonate: basic, combination of a strong base of a weak acid H2CO3 + 2NaOH Na2CO3 + 2H2O Sodium acetate: basic, combination of a strong base and a weak acid CH3COOH + NaOH NaCH3COO + H2O Sodium hydroxide: Neutral, combination of a strong base and a strong acid Na+ + OH- NaOH Sodium chloride: Neutral, combination of a strong base and a strong acid 2HCl + NaOH 2NaCl + H2O Identify amphiprotic substances and construct equations to describe their behaviour in acidic and basic solutions A substance which can act both as a Brönsted-Lowry acid (proton donor) and base (proton acceptor) is called an amphiprotic substance. Amphiprotic substances: H2O, HSO-, HCO3-, H2PO4-, HPO42- Analyse information from secondary sources to assess the use of neutralisation reactions as a safety measure or to minimise damage in accidents or chemical spills Acids and alkalis are corrosive, they can damage skin, clothing and benches. When accidents or spills occur, neutralisation reaction can be utilised to minimise damage. Neutralisation is an exothermic process, therefore it cannot be used for spills on skin as it will cause even more heat and intensify the burn. Spills on floors and benches can be cleaned by neutralisation reaction. Weak base such as sodium hydrogen carbonate NaHCO3 and weak acid such as acetic acid can be added to neutralise the spill follow by a large amount of water. Being a weak acid/base, it won’t produce much heat. Sodium carbonate is widely used to neutralise acidic spills or effluents because: It is a stable solid which is easily and safely handled and stored It is the cheapest alkali available If too much of it is used there is less danger than excess of sodium hydroxide calcium hydroxide. Amphiprotic substances such as sodium hydrogen carbonate can be used to neutralise both acid and base. Never add water to acid but acid to water. Identify neutralisation as a proton transfer reaction which is exothermic Neutralisation reactions are proton transfer reactions which are exothermic with final products as water and a salt. Perform a first-hand investigation and solve problems using titrations and including the preparation of standard solutions, and use available evidence to quantitatively and qualitatively describe the reaction between selected acids and bases Describe the correct technique for conducting titrations and preparation of standard solutions Volumetric analysis is a form of chemical analysis in which the concentration (molarity) or amount of a substance (unknown) is determined by measuring the volume of a solution of known concentration of another substance (the standard) which is just sufficient to react with all of the sample of the first substance Procedure of adding the standard solution to the unknown solution until reaction is completed is called the titration. The point when the reaction is complete is called the equivalence point. With an acid-base titration, there is no colour change and no precipitate formed to show us when the reaction is completed, so an indicator is used. The point at which the indicator changes colour is called the end point. There are a number of indicators available, and they need to be chosen according to the equivalence point. Methyl orange (3.1 – 4.4 pH) Strong acid with weak base Bromothymol blue (6.2 – 7.6 pH) Strong acid with strong base Litmus (5.0 – 8.0 pH) Weak acid with weak base Phenolphthalein (8.3 – 10.0 pH) Strong base with weak acid The equivalence point of acid-base titration can be found by measuring electrical conductance of the solution A standard solution is a solution the concentration of which is accurately known, it is prepared by primary standard, which has the following characteristics: Available at high purity, high molecular mass, unreactive with components of air such as water vapour and carbon dioxide. 1. Wright out appropriate mass of the primary standard into a beaker 2. Add demineralised water until completely dissolved 3. Carefully transfer solution into a volumetric flask, and rinse beaker thoroughly 4. Fill volumetric flask with demineralised water to the mark 5. Stopper flask and invert twenty times to produce homogenous solution Titration Beakers, pipette and burette should be rinsed with the solution it is to deliver Conical flask should be rinsed with demineralised (distilled) water 1. Fill a burette with a solution and adjust the solution level in the burette to the zero mark (ensure no air bubbles) 2. Using the pipette, place a solution of the other sample in a flask under the burette 3. Add two to four drops of a suitable indicator to the flask (indicator is slightly acidic/basic, and thus too much shouldn’t be added) 4. Place a piece of white tile under the flask 5. Run the solution from the burette into the flask with constant swirling rapidly until the end point is close, then run the solution drop by drop carefully until the indictor just changes colour 6. Read the volume (use white background and avoid parallax error) 7. Calculate the required concentration or amount Qualitatively describe the effect of buffers with reference to a specific example in a natural system A buffer solution is a solution which contains comparable amounts of a weak acid(base) and its conjugate base(acid) and which is therefore able to maintain an approximately constant pH even when significant amounts of strong acid or strong bases are added to it. CO2 + H2O H+ + HCO3This is a buffer system in lakes whereby acids and bases are neutralised. When acid is added, carbonic acid is produced, increasing H+ concentration and carbon dioxide escapes into the atmosphere, neutralising the solution due to Le Chatelier’s principle. When hydroxide ion is added, H+ concentration reduced and the reaction shifts to the right to oppose the change. 5. Esterification is a naturally occurring process which can be performed in the laboratory describe the differences between the alkanol and alkanoic acid functional groups in carbon compounds Functional group: an atom, group or group of atoms that reacts in a characteristic way in different carbon compounds Alkanol: -OH hydroxyl group Alkanoic acid: -COOH carboxylic acid group Because of the extra double bonded Oxygen atom alkanoic acid is more polar than alkanol identify the IUPAC nomenclature for describing the esters produced by reactions of straight-chained alkanoic acids from C1 to C8 and straight-chained primary alkanols from C1 to C8 Methanol CH3OH Methanoic HCOOH Ethanol C2H5OH Ethanoic CH3COOH Propanol C3H7OH Propanoic C2H5COOH Butanol C4H9OH Butanoic C3H7COOH Pentanol C5H11OH Pentanoic C4H9COOH Hexanol C6H13OH Hexanoic C5H11COOH Heptanol C7H15OH Heptanoic C6H13COOH Octanol C8H17OH Octanoic C7H15COOH explain the difference in melting point and boiling point caused by straight-chained alkanoic acid and straight-chained primary alkanol structures Alkanoic acid because of the addition polar C=O bond is more polar than alkanoics, therefore from stronger dipole-dipole bonds and hence stronger intermolecular force. Alkanoic acid because of the oxygen also has a higher molecular weight, therefore it has higher melting point and boiling point. identify esterification as the reaction between an acid and an alkanol and describe, using equations, examples of esterification Esterification is the reaction between an acid and an alkanol. Alkanols react with alkanoic acids in a acid-catalysed reaction to form esters. Esters are molecules of strong aroma. describe the purpose of using acid in esterification for catalysis Usually concentrated sulphuric acid is used as a catalyst (18M). It is a dehydrating agent and is used to lower the activation energy, increase the rate of reaction and achieve equilibrium at a much faster rate. explain the need for refluxing during esterification Refluxing is the process of heating a reaction mixture in a vessel with a cooling condenser attached in order to prevent loss of any volatile reactant or product. Esterification is a moderately slow process at room temperature and does not go to completion. Refluxing allows the reaction to be carried out at a higher temperature (near the boiling point of the alcohol) than would otherwise possible. The water-cooled condenser condenses volatile vapour to prevent the loss of both reactant and product, increases the time that reagents are together and allows equilibrium to be reached faster. It also prevents the possibility of an explosion if the reaction is carried out in a closed vessel. outline some examples of the occurrence, production and uses of esters Esters have pleasant, fruity odours and occur widely in nature as perfumes and flavouring agents. E.g. apricot: methyl butanoate orange: octyl ethanoate Esters are a substantial industry for developing and manufacturing synthetic flavours and perfumes. The first step is often to identify the constituents of the natural flavour and then to synthesise similar mixtures of esters which reproduce this flavour. Such artificial flavours are often cheaper than natural extracts, and provide they contain only substances that occur in the natural flavours, the represent little health hazard. Ethyl ethanoate is widely used as a solvent in industry. It is also the common solvent in nail polish remover. High molecular weight (non-volatile) esters such as dialkyl phthalates are used as plasticisers in some plastics such as PVC; they make the materials soft and pliable. Esters are used as artificial perfumes or scents as they emit a sweet smell Esters are used in making artificial food flavours that are added in many edible items like ice creams, soft drinks, sweets, etc Esters are used as industrial solvents for making cellulose, fats, paints and varnishes Esters are used as solvents in pharmaceutical industries Esters are used as softeners in plastic industries and molding industries process information from secondary sources to identify and describe the uses of esters as flavours and perfumes in processed foods and cosmetics If a low molecular weight ester is encountered, the odour is usually pleasant. They are the very popular artificial scents and flavours added to a wide array of consumer products such as soft drinks, chewing gum, scented wax candles, food flavourings, etc. In many cases, although not exclusively so, the characteristic flavours and fragrances of flowers and fruits are due to compounds with the ester functional group. An exception is the case of the essential oils. The organoleptic qualities (odours and flavours) of fruits and flowers are often due to a complex mixture in which a single ester predominates. Food and beverage manufactures are thoroughly familiar with these esters and often use them as additives to spruce up the flavour or odour of a dessert or a beverage. Many times such flavours or odours do not even have a natural basis, as is the case with the juicy fruit principle, isopentenyl acetate. An instant pudding that has the flavour of rum may never have seen its alcoholic namesake – this flavour can be duplicated by the proper admixture, along with other minor components, of ethyl formate and isobutyl propionate. A single compound is rarely used in good-quality imitation flavouring agents. A formula for an imitation pineapple flavour includes ten esters and carboxylic acids that can easily be synthesised in the laboratory. The remaining seven oils are isolated from natural sources. Although the fruity tastes and odours of esters are pleasant, they are seldom used in perfumes or scents that are applied to the body. The reason for this is chemical. The ester group is not as stable to perspiration as the ingredients of the more expensive essential-oil perfumes. The latter are usually hydrocarbons (terpenes), ketones, and ethers extracted from natural sources. Esters, however, are used only for the cheapest toilet waters, since on contact with sweat, they undergo hydrolysis, giving organic acids. These acids, unlike their precursor esters, generally do not have a pleasant odour. Butyric acid, for instance, has a strong odour like that of rancid butter (of which it is an ingredient) and is a component of what we normally call body odour. It is this substance that makes foul-smelling humans so easy for an animal to detect when he is downwind of them. It is also of great help to the bloodhound, which is trained to follow small traces of this odour. Ethyl butyrate and methyl butyrate, however, which are the esters of butyric acid, smell like pineapple and apple, respectively. A sweet fruity odour also has the disadvantage of possibly attracting fruit flies and other insects in search of food. Isoamyl acetate, the familiar solvent called banana oil, is particularly interesting. Fourteen