x/m α P - e-CTLT
advertisement

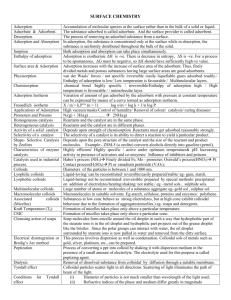
Surface Chemistry Adsorption: It is the phenomenon of attracting & retaining the molecules of a substance on the surface of a liquid or a solid resulting into a higher concentration of molecules on the surface. The substance adsorbed is called adsorbate & the substance on which it is absorbed is called adsorbent. The reverse of adsorption is removal of adsorbed substance from the surface is called desorption. The adsorption of gases on the surface of metals is called occlusion. Cause of adsorption: Adsorption arises at the surface of solids as a result of the presence of unbalanced forces at the surface. Characteristics of adsorption: 1. It is specific & selective in nature. 2. It is accompanied by decrease in the free energy of the system. Since it is a spontaneous process, ▲G is –ve. Absorption: It is a phenomenon in which a substance is uniformly distributed throughout the substance. Adsorption id a surface phenomenon whereas absorption is a bulk phenomenon. Factors on which adsorption of gases on solid depends:1. Nature of adsorbent. 2. Surface area of adsorbent: - Creates the surface area greater in the adsorption. 3. Nature of gas. 4. Temperature: - Adsorption is an exothermic. Therefore increase of temperature decreases the adsorption & vice versa. 5. Pressure: - Adsorption increases with pressure at const. temp. 6. Activation of solid adsorbent: - Activation means increasing the adsorbing power of solid adsorbent. This can be done by removing the gases already adsorbed by heating or by subdividing the adsorbent. Enthalpy of adsorption: - ( heat adsorption) It is the amount of heat evolved when one mole of an adsorbate is adsorbed on the surface of adsorbent. Types of adsorption :1. Physical adsorption 2. Chemical adsorption Physical adsorption : - Particles of adsorbate are held to surface of adsorbent by physical forces such as wander waals forces. Chemical adsorption:- It is a process in which the molecules of adsorbent by chemical forces. Distinction: PHYSICAL CHEMICAL 1. Molecules are attracted to surfaces by Vander Waals forces. 2. Heat of adsorption is low (20-40kj/mol). 3. Process is reversible. Molecules are held by chemical bonds. 4. Decreases with temperature. It first increases then Heat of adsorption is high (40-400kj/mol). Process is irreversible. decreases with temperature. 5. Non-specific in nature. Specific in nature. 6. It forms multi molecular layer. It forms unimolecular. Adsorption isotherm: - It is the plot of the mass of gas adsorbed per gram of adsorbent(x/m) versus equilibrium pressure at const. temp. Freundlieh adsorption isotherm Adsorption of gases on solids: For low pressure, x/m α P For high pressure, x/m α P° For intermediate pressure, x/m α P1/n or x/m = KP1/n n=integer, k=const. Log x/m = log K + 1/n log P Adsorption of solutes from solution by solid adsorbents. x/m = Kc1/n Catalysts:- A substance which can change the speed of a chemical reaction without being used up in the reaction . Types of catalysts: Homogenous catalyst Heterogenous catalyst Homogenous catalyst: When catalyst present is in the same phase as the reactants. Eg:o Lead chamber process for the manufacture H2SO4. 2SO2 + O2 → (cat.=NO) 2SO3 o Hydrolysis of ethyl acetate in the presence of dil.H2SO4 acid. 2CO + O2 → (cat.=NO)2CO2 CH3COOC2H5 +H2O →(cat.=H2SO4) CH3COOH + C2H5OH Heterogenous Catalyst:-When catalyst is present in different phase than that of reactants. Eg:o Manufacture of NH3 using Fe as a catalyst. (by haber’s process) N2+3H2 → (cat. =Fe) 2NH3 CH2=CH2+H2 →(cat.=Pt)CH3-CH3 o Contact process:(Manufacture of H2SO4) SO2+O2→ (cat. =V2O5) SO3 o Polymerization of Ethene using Zeingler-Natta catalyst CH2=CH2→(cat.=TiCl4&Tri.Alkylaluminium)-(CH2-CH2-)n Properties of catalyst: Activity-Activity of a catalyst depends on the strength of chemisorption. The reactant must adsorb reasonably strong strongly for the catalyst to the active. Selective-The ability of a catalyst to direct a reaction to yield a particular product. CO + 3H2 →(cat.=Ni) CH4 + H2O CO + 2H2 →(cat.=Cu/ZnO-Cr2O3)CH3OH CO + H2 →(cat.=Cu)HCHO i.e. a given substance can act as a catalyst only for a particular reaction, not for all the reactions. Eg:- Shape selective Catalysis by Zeolites: Catalytic reaction that depends upon the pore structure of catalysts & the size of reactant & product molecules. Zeolites are good shape selective catalysts. Zeolites are aluminosilicates. Zeolites are 1st heated before used as catalyst to remove water of hydration and create cavities. Size generally varies from 260pm to 740pm this only some molecules can be adsorbed. Eg ZSM-5 converts alcohols to petrol. Enzyme Catalysis: Biochemical catalysts are called enzymes. Enzymes are proteins with high molecular mass. They increase rate by 108 to 1020 times. They are extremely specific. E.g:- Only urease catalyses the hydrolysis of urea & none of the several thousand other enzymes present in the cell catalyses that reaction. NH2CONH2 + H2O → (cat.=urease) 2NH3+ CO2 Enzyme Action:- Two models: Lock & key model Induced fit model or hand in glove model. Lock & key model: Although enzyme is a very large molecule the reaction is catalyzed at a very specific location in the enzyme called active site. The substances that undergo reaction at this site are called substrates. The substrate fits into the active site forming enzyme-substrate complex. Once reaction occurs products depart, allowing another substrate to enter. Induced fit model:The enzyme changes shape when substrate lands at active site. This may be pictured as hand and glove in which the glove (active site) does not attain its functional shape until hand (substrate) moves onto place. COLLOIDS: A colloid is a heterogenous system in which one substance is dispersed (dispersed phase) as every fine particle in another substance called dispersion medium. (Dispersed phase is discontinuous phase whereas dispersion medium is continuous). Colloid particles range in diameter from approximately 10 to 2000Ao. The two phases, dispersed phase & dispersion medium can be solid, liquid or gas. o Sols-solid in liquid colloids. E.g.: starch. o Emulsions- liquids in liquids. E.g.: milk. o Gels- liquids in solids. E.g.: butter, jellies. Aquasol- dispersion medium is water. (Hydrosol) o Alcosol-dispersion medium is alcohol. o Aerosols- dispersion medium is gases. o Benzosols- dispersion medium is benzene. Classification based on interaction between dispersed phase & dispersion medium: LYOPHILIC COLLOIDS: Particles of dispersed phase have a great affinity for the dispersion medium. These are quite stable & cannot be easily coagulated. The solids obtained after evaporation may be reconverted to sol state by simply agitating with dispersion medium. Therefore they are called reversible sol. If water is dispersion medium, it is called hydrophilic. LYOPHOBIC SOLS. :-Particles of dispersed phase have very little or no affinity for dispersion medium. These are less stable & irreversible. Lyophobic sols need stabilizing agents for their preservation. If water is dis. medium, it is called hydrophobic. CLASSIFICATION BASED ON TYPE OF DISPERSED PHASE PARTICLES 1. Multimolecular 2. Macromolecular 3. Associated colloids MULTIMOLECULAR: They are formed by aggregation of large number of atoms or molecules which generally have diameters less than 1nm. E.g.: sols of gold, sulphur, etc. Atom or molecules are held together by weak Wander Waals forces. MACROMOLECULAR:o They are molecules of large size. o E.g. polymers like rubber, nylon, starch etc. when dissolved in suitable solvent from colloidal solution. o They are quite stable. ASSOCIATED COLLOIDS: They are formed by the aggregation of a large no. of ions due to attraction towards oppositely charged ions in concentrated soln. E.g. soap & detergent solns. They behave as normal electrolytes in low conc. but at higher conc. exhibit colloidal behaviour. The aggregate particles thus formed are called micelles. Formation of micelles takes place above a particular temp. called Kraft temperature & above a particular conc. are called critical micelle concentration (CMC). These colloids have both lyophilic & lyophobic parts. MECHANISM OF FORMATION OF MICELLES: E.g. soap. Soap is sodium salt of higher fatty acid RCOO-Na+. E.g. sodium stearate CH3(CH2)16COONa. When dissolved in water it dissociate to form RCOO- & Na+ ion. COO- consists of 2 parts:- Long hydrocarbon chain(R) or non-polar tail which is hydrophloeic & COO- or polar head which is hydrophilic. At higher conc. they aggregate in a spherical form with their R-chain pointing towards centre & COO- part on outward on the surface. PREPARATION OF COLLOIDAL SOLUTIONS: LYOPHOLIC SOLUTIONS: CONDENSATION METHODS DISPERSION METHODS CONDENSATION METHODS (CHEMICAL & PHYSICAL METHODS): Particles of atomic & molecular sizes are induced to combine to form aggregates with colloidal size. o CHEMICAL METHODS: As2O3+3H2S→As2S3+3H2O – double decomposition SO2+2H2S→3S+2H2O – oxidation 2AuCl3+3HCHO+3H2O→2Au+3HCOOH+6HCl – reduction FeCl3+3H2O→Fe(OH)3+3HCl – hydrolysis o PHYSICAL METHODS: Exchange of solvent Excessive cooling 1. Exchange of solvent:- When a true sol. is mixed with excess of other solvent in which the solute is insoluble but solvent is miscible, a colloidal sol is obtained. E.g. solution of sulphur in alcohol is poured in excess of water. – Colloidal sol of sulphur is obtained. 2. Excessive cooling:- The colloidal sol of ice in organic solvent CHCl3 or ether can be obtained by freezing solution of water in the solvent. DISPERSION METHODS:Mechanical dispersion – The substance is brought into a colloidal state in the dispersion medium by grinding it in colloidal mill. or ultrasonic disintegrator. Electrical disintegrator or Bredig’s arc method:In this method electric arc is stuck between electrodes of metal immersed in dispersion medium. The intense heat produced vaporizes the metal which then condenses to form particles of colloidal size. E.g. gold, silver, etc. can be prepared by this method. PEPTIZATION:-In this method precipitate is converted to colloidal sol by shaking it with dispersion medium in the presence of small amount of electrolyte. o The electrolyte used is called peptizing agent. E.g. When Fe(OH)3 is shaken with FeCl3 it absorbs Fe3+ ions & thereby breaksup to smaller sized particles. LYOPHILIC SOLUTIONS:Prepared by shaking lyophilic material with dispersion medium. PURIFICATION OF COLLOIDS:Impurities in sols may destabilize colloids so they have to be removed. METHODS:DIALYSIS OR ELECTROLYSIS ULTRA FILTRATION ULTRA CENTRIFUGATION DIALYSIS: A bag made up of parchment paper is filled with colloidal solution & is then suspended in fresh water. The electrolytes pass out leaving behind the colloidal sol. Only particles of true solution pass through parchment paper not sol particles. ELECTROLYSIS:In this method the movement of ions across the membrane is made faster by applying electric current through two electrodes. ULTRA-FILTRATION: This is the process of separating the particles of colloids from electrolytes by filtration through ultra filter papers. Ultra filter papers allow only electrolytes to pass through. It is prepared by treating ordinary filter paper with gelatine solution to narrow down the pores suitably. ULTRA CONFIGURATION: In this method the colloidal solution is placed in a high speed centrifugal machine. The colloidal particles settle down while impurities remain in the centrifugate. PROPERTIES OF COLLOIDAL SOLUTIONS;o TYNDALL EFFECT:- If a strong beam of light is passed through a colloidal sol the path of the beam gets illuminated. This phenomenon is called TYNDALL EFFECT. o This is due to scattering of light by colloidal particles. BROWNIAN MOVEMENT: The continuous zig-zag movement of colloidal particles in colloidal sol. This is due to unequal impact of particles of dispersion medium on colloidal particles. This phenomenon was observed by Robert Brown in 1827. ELECTROPHORESIS: All the dispersed phase particles in a colloidal solution carry same charge (either +ve or –ve) while dispersion medium has equal & opp charge. Particles therefore repel each other & do not come together to form large particles. E.g.As2S3, Au, Ag, Pt are –vely charged. Fe(OH)3 , Al(OH)3 are +vely charged. The charge on colloidal particles in due to preferential adsorption of ions. E.g. Fe(OH)3 adsorbs Fe3+ ions from FeCl3 soln. AgCl by adsorption of Cl- ions. Existence of charge can be shown by electrophoresis which involves the movement of colloidal particles either towards cathode or anode under the influence of electric field. COAGULATION VALUES:-The minimum amount of an electrolyte that must be added to one liter of colloidal soln. so as to cause its complete coagulation is called coagulation value. HARDY-SCHULZE RULE: The ions carrying charge opposite to that of sol particles are effective in causing the coagulation of sol. Coagulating power of an electrolyte is directly proportional to the fourth power of valency of ions causing coagulation. E.g.Fe3+ is more effective than B2+ for coagulation of As2S3 soln. PO43- is more effective than SO 42- or d-ions for coagulation of Fe(OH)3 sol. EMULSIONS:- are colloids in which both dispersion phase & dispersion medium are liquids. o OIL IN WATER EMULSIONS:-Oil acts as dispersion phase and water acts as dispersion medium. o E.g. old cream, butter. IDENTIFICATION OF EMULSIONS: TESTS:I. DILUTION TESTS:-If emulsion can be diluted with water, this indicates that dispersion medium is water i.e. it is oil in water type. If added water forms a separate layer it is water in oil type. II. DYO TEST:- Some oil soluble dye is added to the emulsion if the background becomes colored, emulsion is water-in oil type, & if colored droplets are seen emulsion is oil in water type. PREPARATION OF EMULSIONS :EMULSIFICATION: Process of making an emulsion is known as emulsification. To stabilize emulsion substances added are called emulsifying agents or emulsifier. E.g. soap & detergents. DEMULSIFICATION:Separation of an emulsion into its constituent liquids is called demulsification. o Methods used are – boiling, freezing, centrifugation, etc. APPLICATION:a) Rubber plating b) Sewage disposal c) Cottrell smoke precipitator d) Preparation of nano materials e) In medicines f) In disinfectants g) In metallurgical operations.