Atomic Structure & Periodic Table: Textbook Excerpt
advertisement
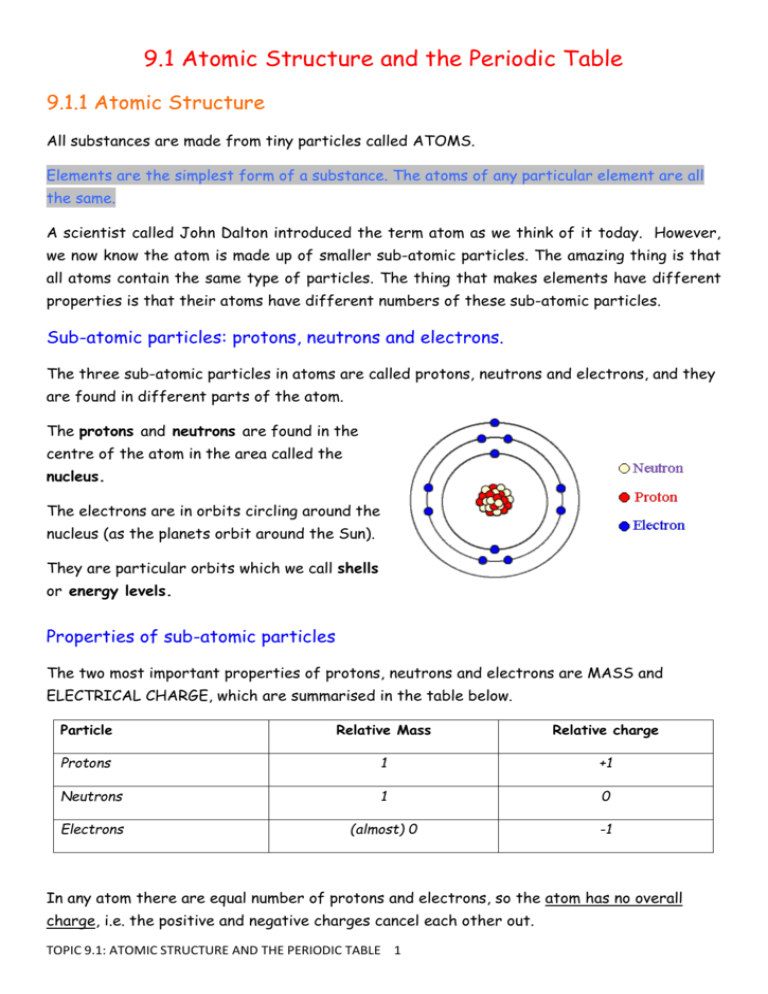
9.1 Atomic Structure and the Periodic Table 9.1.1 Atomic Structure All substances are made from tiny particles called ATOMS. Elements are the simplest form of a substance. The atoms of any particular element are all the same. A scientist called John Dalton introduced the term atom as we think of it today. However, we now know the atom is made up of smaller sub-atomic particles. The amazing thing is that all atoms contain the same type of particles. The thing that makes elements have different properties is that their atoms have different numbers of these sub-atomic particles. Sub-atomic particles: protons, neutrons and electrons. The three sub-atomic particles in atoms are called protons, neutrons and electrons, and they are found in different parts of the atom. The protons and neutrons are found in the centre of the atom in the area called the nucleus. The electrons are in orbits circling around the nucleus (as the planets orbit around the Sun). They are particular orbits which we call shells or energy levels. Properties of sub-atomic particles The two most important properties of protons, neutrons and electrons are MASS and ELECTRICAL CHARGE, which are summarised in the table below. Particle Relative Mass Relative charge Protons 1 +1 Neutrons 1 0 Electrons (almost) 0 -1 In any atom there are equal number of protons and electrons, so the atom has no overall charge, i.e. the positive and negative charges cancel each other out. TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 1 9.1.2 The atomic number How do we know how many sub-atomic particles an atom has? The answer to this is in the Periodic Table. Each element has 2 numbers written next to their symbol. The smaller number is called the Atomic number and tells us the number of protons in the atoms of that element. It is also known as the Proton Number. Atomic number = number of protons For example, consider the elements sodium (Na), sulphur (S) and neon (Ne). 23 32 20 11 16 10 Na S Ne The atomic number for each of these elements is 11 for sodium, 16 for sulphur and 10 for neon. The atomic number is always the same for that element. So if we are told the number of protons we can always identify the element using our Periodic Table. Sometimes the Periodic Table has only one number present next to the symbol, in these versions it is the Atomic Number as in the copy on p24 of the AQA text book. The atomic number increases from top to bottom and left to right in the table. Another useful thing about the atomic number… For any neutral atom the number of protons equals the number of electrons. So, the atomic number can tell us about the electrons as well. For example, the atomic number of oxygen is 8, so it has 8 protons and 8 electrons. 9.1.3 The mass number The larger number is called the mass number and is the sum of the number of protons and neutrons in the nucleus. The mass of any atom is simply all of the protons and neutrons added together as each one is adding 1 unit to the mass of the atom. Mass number = number of protons + number of neutrons Therefore, number of neutrons = mass number - number of protons. For example, the number of neutrons in sodium, sulphur and neon can be found using the information and the equations given above: Sodium: Number of neutrons = 23 - 11 = 12 Sulfur: number of neutrons = 32 - 16 = 16 Neon: number of neutrons = 20 - 10 = 10. TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 2 9.1.4 Electron shells and electron configuration Electrons in atoms occur in shells (shown as circles around the nucleus). The shells can also be called energy levels. The maximum number of electrons in each shell, going from the middle to the outside, is 2, 8, 8, 18. Below is a picture of a potassium atom. The atomic number of potassium is 19. This means it has 19 protons, and so also has 19 electrons in total. The 19 electrons are arranged around the nucleus in the following way: 1st shell: 2nd shell: 3rd shell: 4th shell: 2 electrons 8 electrons 8 electrons 1 electron The electron configuration (also known as the electron structure) of potassium is therefore written as 2,8,8,1. In Year 9 you will need to learn the electron configurations of the first 20 elements in the Periodic Table (hydrogen to calcium). To work out the configurations, you need to establish how many electrons an atom of the element has in total, and then arrange them in the shells, making sure not to overfill any particular shell. Once a shell is full and you still have more electrons to put in, you put the extra electrons into the next shell outwards from the middle until you run out of electrons. For example, take lithium (Li). It has an atomic (proton) number of 3. It therefore has 3 electrons that orbit the nucleus. The first two electrons go in the first shell. The third electron cannot be put into this shell as it’s full, so it must go into the next shell out. The electron configuration of a lithium atom is therefore 2,1. An atom which has the maximum number of electrons in its outer shell will be stable. It has achieved the electronic structure of a noble/inert gas and this means that it will not react with other atoms. If the outer shell of an atom has less than its maximum number of electrons (see potassium above) then it will not be stable. It will react with other atoms to get a full outer shell. The number of electrons in the outer shell of an atom is the same as the group number on the Periodic Table, so all elements in the same group will have similar chemical properties. Lithium, sodium and potassium all have one electron in their outer shell and all react in a similar way. TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 3 9.1.5 The Periodic Table Scientists called Newland and Mendeleev attempted to classify the elements by arranging them in order of their atomic weights or masses. Newland was known for arranging his periodic table by atomic mass and discovering then that every eighth element seemed to have similar properties. This was know as ‘Newland’s Octaves’ (as an octave is every eighth note in music). The list can be arranged in a table so that elements with similar properties are in columns known as Groups. The table is called a Periodic Table because similar properties occur at regular intervals. However, it did not work all of the time and unreactive metals like copper (Cu) were put in the same group as very reactive metals like potassium (K). In other groups, very reactive gases were placed with low reactivity metals. You can also note that in Newlands’ table there were no noble gases because they had not been discovered at that time. Also, Newlands had to place two elements in the same position in his periodic table in order to make it work. The early Periodic Tables were incomplete and some elements were placed in inappropriate Groups if the strict order of atomic weights were followed. The Russian scientist Dmitri Mendeleev overcame some of the problems by leaving gaps for the elements that he thought had not been discovered. Not only that, he was able to predict that these elements would be discovered in the future and also what the properties of these undiscovered elements would be based on the known properties of elements already in that group or period. This was an early attempt of Mendeleev’s and his ideas would be improved upon years later. TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 4 In 1871 Mendeleev predicted the properties of what we now call Germanium (Ge) in group IV. This element was discovered 15 years later and its properties where almost exactly as Mendeleev had said they would be. Appearance Density (g/cm3) Relative atomic mass Melting point (oC) Reaction with oxygen Predicted element Germanium (Ge) Grey metal 5.5 73.4 ~800 Might form MO2 and MO Grey-white metal 5.47 72.6 958 Forms GeO2 and GeO The modern Periodic Table arranges elements by Atomic Number (ie. the number of protons in the atom) not by Atomic Mass, although it has a similar pattern for most of the elements. However, if you look carefully you can spot where arranging by mass would place an element in the wrong Group and its properties would not follow the trend. For example, look at element 52, Tellurium (mass 128) and then element 53, Iodine (mass 127…it is less than the mass of Tellurium!). This is often questioned in GCSE exams where they expect you to realise the order in Atomic Number and that gaps were present in early forms of the table because elements had yet to be discovered. The modern table also can be seen as an arrangement of elements in terms of their electronic structures. Elements in the same group have the same number of electrons in their outer shell. As you learn about bonding you will see how the number of electrons in the outer shell and how far they are from the nucleus is the factor that governs how elements react. Group 1: The Alkali Metals (Li, Na, K, Rb, Cs) The elements in Group 1 of the Periodic Table are known as the Alkali Metals. They: are metals with low density (the first 3 elements in Group 1 are less dense than water) react with non-metals to form ionic compounds in which the metal ion carries a charge of +1. The compounds are white solids that dissolve in water to form colourless solutions react with water releasing hydrogen form hydroxides that dissolve in water to give alkaline solutions. In Group 1, the further down the Group an element is: the more reactive the element the lower its melting and boiling point TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 5 Group 7: The Halogens (F2, Cl2, Br2, I2, At2) The elements in Group 7 of the Periodic Table (known as the Halogens): have coloured vapours consist of molecules that are made up of pairs of atoms form ionic salts with metals in which chloride, bromide or iodide ions (halide ions) are formed. They carry a charge of -1 form molecular compounds with other non-metallic elements In Group 7, the further down a group an element is: the less reactive the element the higher its melting point and boiling point A more reactive halogen can displace a less reactive halogen from and aqueous solution of its salt. The Transition Elements In the Periodic Table between Groups 2 and 3 is a block of elements known as the transition elements. These elements are all metals. Compared with the elements in Group 1, transition elements: have higher melting and boiling points (except for mercury) and higher densities are stronger and harder are much less reactive and so do not react as vigorously with water and oxygen Many transition elements have ions with different charges, form coloured compounds and are useful as catalysts. The inert gases (noble gases) The elements in Group 8 or 0 are known as the inert gases as they are unreactive or inert. This is due to them having full outer shells of electrons so they do not try to bond with other elements to fill them. They are all gases that exist as monatomic forms (ie. as one atom, not grouped in a molecule like the halogens F2, hydrogen H2 or oxygen O2). TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 6 TOPIC SUMMARY Atoms are tiny particles which are made up of __________ and __________ in the nucleus, with ____________ in shells around the outside. Protons have a _________ charge whilst electrons have a ________ charge. In a neutral atom the number of protons and electrons is __________. All of the mass of an atom is in the _______ because the mass of an electron is almost zero. 23 Na has _____ protons and _____neutrons. __________ are atoms of the same element which have different numbers of neutrons. The electrons in an atom of 23 Na are arranged 2,8, __ . All elements in group 2 of the periodic table have _____ electrons in their outer shell. Scientists originally arranged the periodic table in order of atomic ________ but today’s periodic table is arranged in order of atomic ________. Mendeleev is regarded as the father of the modern periodic table. He left _______ in his periodic table for elements which had not yet been ___________ and he even made ___________ about the properties of these missing elements. gaps negative 1 11 positive protons isotopes electrons equal 13 neutrons TOPIC 9.1: ATOMIC STRUCTURE AND THE PERIODIC TABLE 7 2 predictions discovered mass number nucleus






