Bonding practice (1)
advertisement
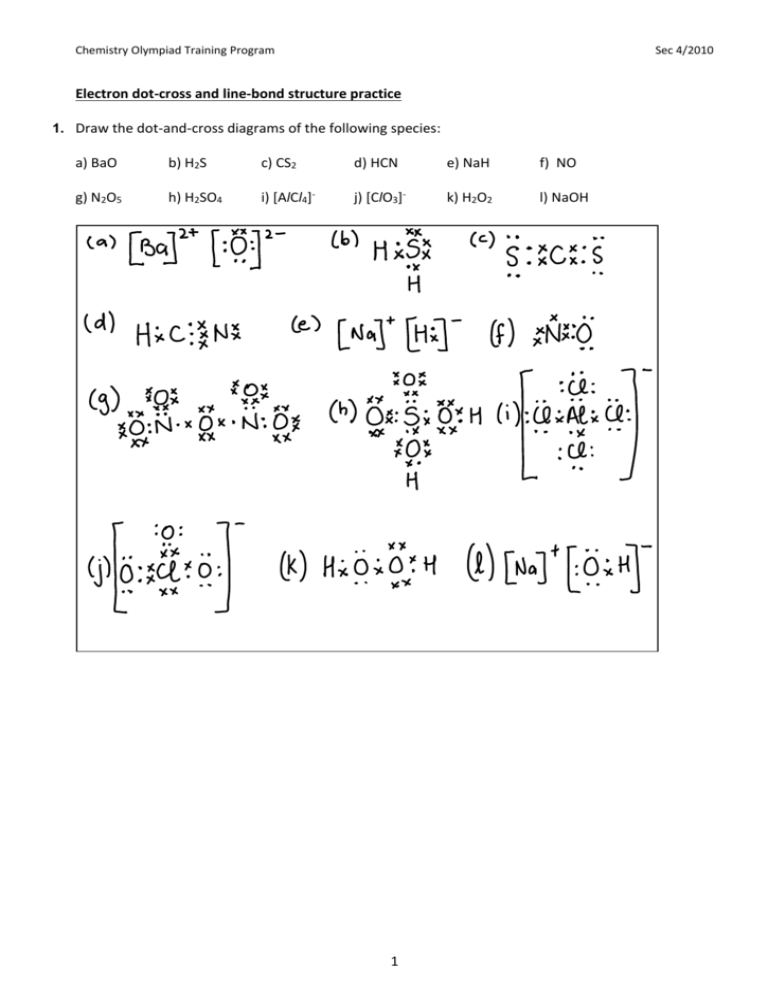
Chemistry Olympiad Training Program Sec 4/2010 Electron dot-cross and line-bond structure practice 1. Draw the dot-and-cross diagrams of the following species: a) BaO b) H2S c) CS2 d) HCN e) NaH f) NO g) N2O5 h) H2SO4 i) [AlCl4]- j) [ClO3]- k) H2O2 l) NaOH 1 Chemistry Olympiad Training Program Sec 4/2010 2. Draw a line-bond structure for each of the following: a) SiF4 b) SeCl2 c) COF2 d) PH4+ e) C2F2 f) SbH3 g) HNO3 h) IF5 i) HCO3- j) AsF4 k) BeCl2 l) XeF4 2 Chemistry Olympiad Training Program Sec 4/2010 3. Draw line-bond structures for the following nitrogen oxides. (a) NO (b) NO2 (c) N2O (d) N2O4 (e) N2O5 4. Discuss the nature of the bonding present in solid NH4Cl. Solid NH4Cl is an ionic compound made up NH4+ cations and Cl– anions. Within the NH4+ cations, each H atom is covalently bonded to the central N atom in a tetrahedral shape. One of the N-H bonds is a dative bond (from the N to the H). The overall NH4+ ion carries a positive charge as the N atom has ‘lost’ one of its original electrons to the chlorine atom. 5. It is known that AlCl3 and N(CH3)3 react readily to form an adduct. [An adduct is the result of the direct combination of 2 separate molecules]. a) Explain why these two molecules form a product when they react in a molar ratio of 1:1. The Al atom in AlCl3 is electron deficient, lacking 2 electrons (i.e. one pair) in order to achieve full octet. The N atom in N(CH3)3 has one lone pair of electrons. The two molecules can react by forming a single dative bond between the N atom on N(CH3)3 and the Al atom on AlCl3. This has to be in a 1 to 1 ratio. b) Draw the dot-and-cross diagram and describe the shape of the adduct. The adduct will be tetrahedral with respect to the N and Al atoms, as each now has 4 bond pairs. 3 Chemistry Olympiad Training Program Sec 4/2010 6. Molten beryllium chloride reacts with chloride ion from molten NaCl to form the BeCl42- ion, in which the Be atom attains an octet. Show the ionic reaction with line-bond structures. 7. *Question to be handed up* AlF3 has high melting point of 1275oC and AlCl3 has melting point of only -150oC. a) Draw the dot and cross diagrams to illustrate the bonding in AlCl3 and AlF3. b) Explain the difference in the melting point between AlCl3 and AlF3. AlCl3 is a simple covalent molecule with relatively weak intermolecular dispersion forces. These require little heat energy to overcome, hence the melting point of aluminium chloride will be low. On the other hand, AlF3 is an ionic compound composed of oppositely charged ions held together by strong electrostatic forces of attraction in a giant lattice structure. These forces require more energy to overcome, hence AlF3 will have a higher melting point. c) State the difference in one other physical property (besides melting point and boiling point) for the two compounds, AlCl3 and AlF3. AlCl3 does not conduct electricity at room temperature (it is a gas) or in molten state. AlF3 is ionic and hence conducts electricity in molten state. d) Aluminium chloride catalyses certain reactions by forming carbocations, C 2H5+, with chloroethane as shown in the following equation. C2H5Cl + AlCl3 C2H5+ + AlCl4Explain, in terms of bonding, how AlCl4- is formed. The C-Cl bond breaks with both electrons going to the Cl, thus a Cl – ion is formed. The Al in AlCl3 is electron deficient. Hence it can accommodate an additional pair of electrons, which can be given by the chloride ion via a dative bond. This forms the AlCl4- ion. 4 Chemistry Olympiad Training Program Sec 4/2010 Shapes Challenge 8. For each of the following species, deduce the shape and bond angles: (a) SO2 (b) NCl3 (c) XeF3+ (d) SbF6– (e) AlCl3 (f) NH4+ (g) BeCl2 (h) SnCl2 (i) PCl5 (j) BrCl5 (k) O3 (l) PH3 (m) ICl4– (n) SO3 (o) Cl2O (p) HNO3 (q) H3O+ (r) ClF4– (s) SF4 (t) BrF3 (u) NO2 (v) N 2O (w) N2O4 (x) N2O5 (y) SbF5 (z) I3– 9. Deduce the shape and bond angles in the following compounds of Xenon: (a) XeF4 (b) XeOF2 (e) XeO3F2 (f) XeO3 (c) XeOF4 (d) XeO2F2 10. Deduce the shape and bond angles in the following organic molecules: (a) CH3OH (b) N(CH3)3 (c) C2H4 (d) H2CO (e) CCl2F2 (f) C2H6 (g) C2H2 (h) CH3CO2H 11. AlCl3 is an electron deficient species. In the vapour phase, the molecule resolves this deficiency by forming dimers, Al2Cl6. Suggest the structure of the dimer, stating its shape and bond angles. 5