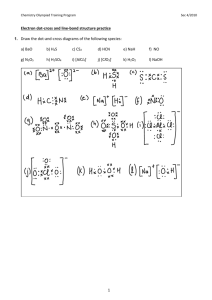
Precipitation Reactions Practice Problems – Set 1
advertisement
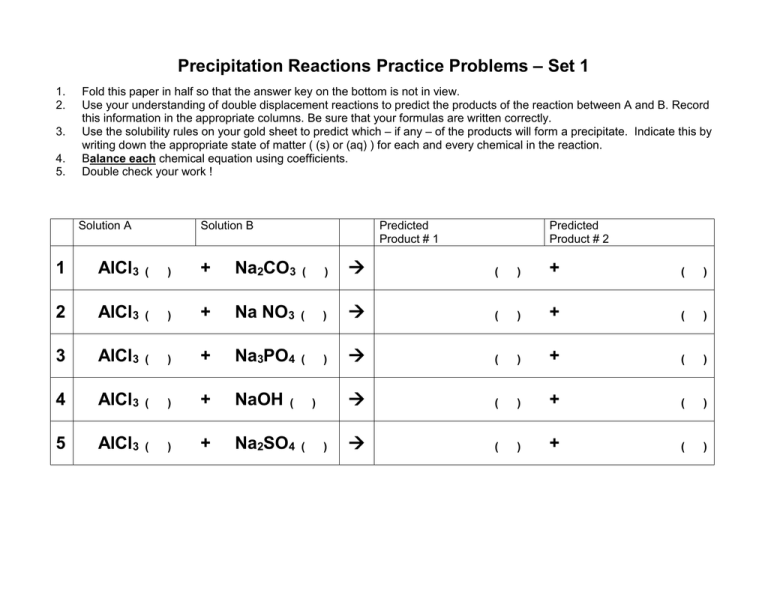
Precipitation Reactions Practice Problems – Set 1 1. 2. 3. 4. 5. Fold this paper in half so that the answer key on the bottom is not in view. Use your understanding of double displacement reactions to predict the products of the reaction between A and B. Record this information in the appropriate columns. Be sure that your formulas are written correctly. Use the solubility rules on your gold sheet to predict which – if any – of the products will form a precipitate. Indicate this by writing down the appropriate state of matter ( (s) or (aq) ) for each and every chemical in the reaction. Balance each chemical equation using coefficients. Double check your work ! Solution A Solution B Predicted Product # 1 Predicted Product # 2 1 AlCl3 ( ) + Na2CO3 ( ) ( ) + ( ) 2 AlCl3 ( ) + Na NO3 ( ) ( ) + ( ) 3 AlCl3 ( ) + Na3PO4 ( ) ( ) + ( ) 4 AlCl3 ( ) + NaOH ( ) + ( ) 5 AlCl3 ( ) + Na2SO4 ( ) + ( ) ( ) ( ) Answer Key - Precipitation Reactions Practice Problems – Set 1 Solution A Predicted Product # 1 Solution B 1 2 AlCl3 (aq ) + 3 Na2CO3 (aq) 2 AlCl3 (aq) + 3 NaNO3 (aq ) Al(NO3)3 3 AlCl3 (aq ) + Na3PO4 (aq) AlPO4 4 AlCl3 (aq ) + 3 NaOH (aq) Al(OH)3 5 2 AlCl3 (aq ) + 3 Na2SO4 (aq) Al2(SO4)3 Al2(CO3)3 Predicted Product # 2 (s) + 6 NaCl (aq ) ( aq ) + 3 NaCl (aq ) + 3 NaCl (aq ) (s) + 3 NaCl (aq ) ( aq ) + 6 NaCl (aq ) (s)