doc - Michael A. Repucci
advertisement

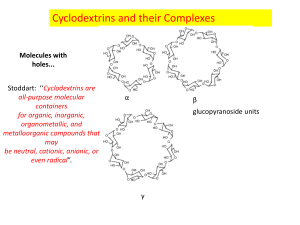
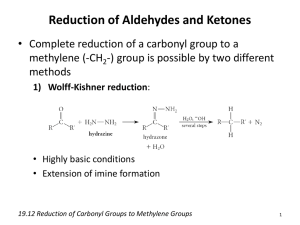
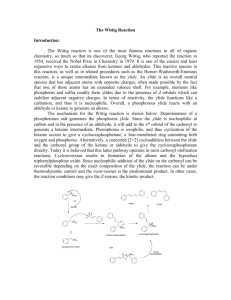
An Alternate Synthesis of 6A, 6B-Dideoxy6A, 6B-(9,10-Dicyanoanthracenyl-2, 3-Dimethylene)-Cyclodextrin ____________ A Thesis Presented to the Faculty of The Department of Chemistry at The College of William and Mary In Partial Fulfillment of the Requirements for the Degree of Bachelor of Science ____________ by Michael A. Repucci May 4th, 1998 Approval Sheet This thesis is submitted in partial fulfillment of The requirements for the degree of Bachelor of Science Michael A. Repucci Approved May 4th, 1998 _______________________ Christopher J. Abelt, Ph. D. _________________________ Carey K. Bagdassarian, Ph. D. ______________________ Deborah C. Bebout, Ph. D. _______________________ Harvey J. Langholtz, Ph. D. Table of Contents ACKNOWLEDGEMENTS ........................................................................................................... iv LIST OF FIGURES .........................................................................................................................v ABSTRACT ................................................................................................................................... vi INTRODUCTION..........................................................................................................................2 BACKGROUND ............................................................................................................................3 Cyclodextrins ...................................................................................................................................3 Dess-Martin Periodinane .................................................................................................................7 Dicyanoanthracene ...........................................................................................................................9 The Wittig Reaction .......................................................................................................................10 EXPERIMENTAL .......................................................................................................................15 Dess-Martin Periodinane ...............................................................................................................15 Polyformyl -CD ...........................................................................................................................16 2, 3-Bis(bromomethyl)-9, 10-dicyanoanthracene ..........................................................................16 9, 10-Dicyanoanthracenyl-2, 3-bis(methyltriphenylphosphonium bromide) ................................17 Polyformyl-6A, 6B-dideoxy-6A, 6B-(9,10-dicyanoanthracenyl-2, 3-dimethylene)--CD ..............18 RESULTS AND DISCUSSION ..................................................................................................19 CONCLUSION ............................................................................................................................23 REFERENCES .............................................................................................................................26 VITA..............................................................................................................................................28 iii Acknowledgements I would like to sincerely thank Dr. Christopher J. Abelt for having patience with my irregular schedule and my endless questions, and for his nonchalant approach and witty sense of humor. I am further indebted to Dr. Deborah C. Bebout, who tolerates my absent mindedness and frequent interruptions. I would also like to thank Ahmed Hafez for his expert advice and assistance, and his perpetual facetiousness. I offer my gratitude to Peggy Green for handling my numerous administrative requests. And I am grateful to all my friends and family who have always provided stress relief and helped to keep me sane in this insane world. iv List of Figures 1. Nomenclature and bonding in cyclodextrin ................................................................................3 2. Supramolecular geometry of cyclodextrin ..................................................................................4 3. Inclusion complex formation and the dissociation constant, Kd .................................................5 4. An example of A, B cap notation of -CD .................................................................................6 5. Photocatalytic cyclodextrin derivatives from the literature ........................................................7 6. The synthesis of DMP .................................................................................................................7 7. Polyformyl cyclodextrin regioisomerism ...................................................................................9 8. 9, 10-Dicyanoanthracene (DCA) ................................................................................................9 9. The fluorescence quenching of 6A, 6D/C-(DCA-2, 6-disulfonyl)--CD ....................................10 10. Phosphonium ylide resonance structure..................................................................................11 11. Preparation of the Wittig reagent ............................................................................................11 12. General mechanism for the Wittig reaction ............................................................................13 13. Tethered and capped DCA--CD ...........................................................................................14 14. The synthesis of 9, 10-dicyano-2, 3-bis(bromomethyl)anthracene ........................................17 15. The oxidation of -CD using 1.5 equivalents of DMP ...........................................................20 16. The synthesis of the bis-phosphonium ylide ...........................................................................21 17. 1H NMR spectrum of polyformyl--CD .................................................................................24 18. 1H NMR spectrum of 2, 3-bis(bromomethyl)-9, 10-dicyanoanthracene ................................25 v Abstract 6A, 6B-Dideoxy-6A, 6B-(9,10-dicyanoanthracenyl-2, 3-dimethylene)--cyclodextrin was synthesized via the Wittig reaction of polyformyl -cyclodextrin and the ylide of 9,10dicyanoanthracenyl-2, 3-bis(methyltriphenylphosphonium bromide), followed by reduction with sodium borohydride. The -cyclodextrin cavity was effectively lengthened as a result of the perpendicular position of the dicyanoanthracene cap to the primary face of the -cyclodextrin. The two olefinic bonds of the cap also help to increase the rigidity of the -cyclodextrin. The resulting molecule contains the oxidative features of dicyanoanthracene in the context of the stereospecific -cyclodextrin cavity. vi An Alternate Synthesis of 6A, 6B-Dideoxy6A, 6B-(9,10-Dicyanoanthracenyl-2, 3-Dimethylene)-Cyclodextrin Introduction Catalysts are molecules that are able to increase the rate of reactions, by providing a more energetically feasible pathway from reactants to products, without being consumed. The use of catalysts is widespread in chemistry, but few synthetic catalysts demonstrate the efficiency and specificity of natural catalysts, or enzymes. Therefore much scientific research is aimed at understanding the mechanisms behind biological enzyme-substrate catalysis. By mimicking natural systems, researchers have developed many synthetic chemical catalysts whose applications include drug delivery macromolecules, fermentation methods, and the simplification of organic synthesis.1 Photocatalysis is a particularly useful methodology for organic chemical synthesis, allowing scientists to use light, rather than excessive heat that may degrade reactants or products, to drive particular reactions. Photooxidative catalysis requires the photon-induced excitation of an acceptor molecule electron from its ground state to an excited state, and subsequent electron transfer or hydrogen abstraction from a donor molecule, thus leaving the donor oxidized and returning the acceptor to its ground state. Many photooxidative catalysts are known, including anthraquinone, benzophenone, and 9,10-dicyanoanthracene (DCA); however, they are often plagued with inefficiency and a lack of regio- or stereospecificity.1 Cyclodextrins, cyclic oligomers of glucose, participate in regio- and stereospecific catalysis. Not surprisingly, there are several examples in the literature where photocatalytic moieties have been attached to cyclodextrins, thus producing photocatalysts with regio- and stereospecificity.2 The goal of this project is to attach DCA by a relatively simple method to cyclodextrin (-CD), thereby forming a novel regio- and stereospecific photocatalyst, DCA capped -CD. 2 Background Cyclodextrins Villiers first discovered cyclodextrins, also called Schardinger dextrins, cycloamyloses, or cyclogulcans, in 1891 as byproducts of the action of Bacillus macerans amylase on starch. In the 1920’s Schardinger developed a detailed method for the preparation and separation of cyclodextrins, which takes advantage of the differing solubilities of cyclodextrin oligomers, using organic solvents to induce the selective precipitation or solvation of particular oligomers of cyclodextrin. With the discovery later in this century that cyclodextrins are able to form inclusion complexes (i.e. host-guest non-covalent interactions similar to enzyme-substrate complexes), and act as catalysts that mimic enzymes in their regio- and stereospecificity, interest in these organic molecules has increased.2 Recent industrial applications are ubiquitous: everything from the chemistry of food, pharmaceuticals, cosmetics, pesticides, and polymers.3 Cyclodextrins are cyclic oligomers of -(1, 4)-linked D-(+)-glucopyranose units in the shape of a torus. The preferred chair conformation of glucose is virtually undisturbed in cyclodextrins. The nomenclature derived for convenience makes reference to the number of interconnected glucose units; the prefixes , , , and denote cyclodextrin molecules consisting OH O of 6, 7, 8, and 9 glucose units, respectively (see Figure 1). OH OH O O HO O HO OH O O n = 5 ... -cyclodextrin HO OH O O HO OH n = 7 ... -cyclodextrin HO O OH n = 8 ... -cyclodextrin OH -CD n = 6 ... -cyclodextrin O OH OH O OH HO O O GLUCOSEn OH HO O OH O OH Figure 1. Nomenclature and bonding in cyclodextrin 3 OH OH HO O O Cyclodextrins of fewer than six glucose units are too sterically strained to exist while those greater than eight units are more challenging to synthetically create and isolate. To date, -, -, -, and -cyclodextrin have been successfully isolated in good yields.2 The supramolecular geometry of cyclodextrins resembles a truncated cone with two openings of different diameters (see Figure 2). The smaller opening, or primary face, consists of primary hydroxyl groups at the C6 position of the glucose, while the secondary face contains secondary hydroxyls at the C2 and C3 positions. The dimensions of -CD (MW 1135 g/mol) are 6.0 Å and 6.8 Å at the primary and secondary face, respectively, and 8.0 Å in height. The C6 hydroxyls are free to rotate, effectively blocking the opening of the primary face, or readily substituted with various functionalities. The presence of opposing C-H bonds, hydrogen-bonded C2 and C3 hydroxyls, and the ether-like oxygens that connect adjacent glucose units, give rise to a relatively hydrophobic inner cavity. The well-defined size and features of -CD contribute to its regio- and stereospecificity. As a result, -CD serves as an ideal enzyme model.2 6.0 Å 8.0 Å 6.8 Å Figure 2. Supramolecular geometry of cyclodextrin Based on the characteristics of cyclodextrin, it is not surprising that it readily forms inclusion complexes with virtually any small molecule, usually in a 1:1 ratio. For instance, in dimethyl sulfoxide (DMSO), -CD forms an inclusion complex with a single water molecule in which C2 and C3 hydroxyls participate in strong hydrogen bonding, both inter- and 4 intramolecularly. Cyclodextrins are very soluble in water, relatively stable in basic solutions, but susceptible to hydrolysis in acids. In some instances only one of two enantiomers efficiently forms an inclusion complex with cyclodextrin, thus permitting a crude, but simple separation of the D- and L-isomers.2 Dissociation constants (Kd) for cyclodextrin inclusion complexes are often desired, and must be obtained empirically according to the relationship below (see Figure 3). The most common empirical method is 1H NMR, which can be used to determine the relative amounts of the host-guest complex and the dissociated host and guest by integrating the respective peaks. Other types of spectroscopy have used the same relationship between peak areas to determine Kd, including 13C NMR, fluorescence intensity, and FTIR.2 + O O O O Kd = Kd [host][guest] [host-guest] Figure 3. Inclusion complex formation and the dissociation constant, Kd There are two primary classifications for the types of catalysis that cyclodextrins can perform. Non-covalent catalysis occurs when a molecule forms an inclusion complex with cyclodextrin, permitting the molecule then to undergo chemical rearrangement that was otherwise impossible in the surrounding solvent. Covalent catalysis begins with non-covalent inclusion complex formation, but bond cleavage or rearrangement is coordinated between both the cyclodextrin host and the guest molecule. If a compound such as pyridine is used in excess to block the cyclodextrin cavity, the desired inclusion complex fails to associate, and catalysis is effectively prevented.2 5 Many functional groups can be covalently attached to the cyclodextrin hydroxyl groups, thereby altering the catalytic properties of the cyclodextrin. In 1994 Breslow and Zhang developed a cyclodextrin dimer that catalyses the cleavage of phosphate esters.4 The attachment of substituents by one bond to either the primary or secondary face of cyclodextrin is referred to as a tether. When a substituent is attached to two points on a face of cyclodextrin, then the functionality is termed a cap. To date, caps have only been attached to the primary face of cyclodextrin. Glucose units are consecutively labeled to indicate the points of attachment to the G cap (see Figure 4). A OH O OH O O O HO O F HO OH O OH O HO OH O X HO O B O OH OH HO O O OH E HO OH OH HO O OH O O C O OH D Figure 4. An example of A, B cap notation of -CD The syntheses of numerous photochemically active derivatives of cyclodextrin appear in the literature. In 1986 Neckers and Paczkowski reported the synthesis of a rose bengal tethered -CD that has the capability of photooxidizing 1,2-diphenyl-p-dioxenes.5 Six years later, Ye et al. synthesized a flavin tethered -CD that photocatalyzes the oxidation of substituted benzyl alcohols to their corresponding aldehydes.6 Then in 1993 Kuroda and coworkers described the photoreduction of quinones, through electron transfer, by utilizing a cyclodextrin-sandwiched porphyrin (see Figure 5).7 6 CH3 N ONa CH2O2C(CH 2)4CH2O2C NH N H2C O N O ROSE BENGAL PORPHYRIN O Figure 5. Photocatalytic cyclodextrin derivatives from the literature Dess-Martin Periodinane The Dess-Martin Periodinane (DMP) is a useful reagent for the selective oxidation of primary and secondary hydroxyls to aldehydes and ketones. The original synthesis of DMP, unfortunately, gives relatively low yield.8 However, Ireland and Liu have developed a method that results in pure DMP in excellent yield; the synthesis is outlined below (see Figure 6).9 DMP is very hygroscopic and therefore must be protected from exposure to atmospheric moisture. Hydrolysis of DMP occurs readily at room temperature yielding the intermediate seen in figure six, which the authors caution is explosive similar to trinitrotoluene (TNT), detonating as a result of excessive heating (>200 ºC) or impact.8, 9 OAc O CO2H I KBrO3 H2SO4(aq) O O O I OH O Ac2O 0.5% TsOH I OAc OAc Figure 6. The synthesis of DMP Since its development, DMP has found widespread use in the oxidation of alcohols, thereby virtually replacing the need for chromium (VI) reagents. Dess and Martin studied the reaction of DMP with many alcohols (benzylic, allylic, or otherwise) and found consistently high yields for the oxidation of alcohols in the presence of non-hydroxylic functional groups such as 7 sulfides, enol ethers, furans, and secondary amines. The reaction of DMP with a variety of alcohols is rapid and essentially quantitative at room temperature; acetaldehyde is produced from excess ethanol, cyclooctanal is afforded in 86% yield from cyclooctanol with 1.15 equivalents (eq) of DMP, geranial is provided in 84% yield from geraniol with 1.1 eq of DMP, etc. Dess and Martin site 74 papers that describe useful oxidations by DMP.8 Many researchers have found DMP to be a useful oxidant in the production of synthetic biomolecules. Hanson and Lindberg report the use of DMP in the production of dipeptide analogues containing novel ketovinyl functionalities that are useful as mechanism-based enzyme inhibitors and proteolytically stable peptides.10 In 1988 Danishefsky et al. began using DMP in the synthesis of enediyne antitumor antibiotics.11 Eight years ago, Robins and coworkers used DMP for the preparation of ketonucleosides, analogues of the building blocks for DNA.12 DMP also provides a simple method for the oxidation of the primary alcohols of cyclodextrin in high yield, furnishing formyl cyclodextrin, thereby simplifying the synthesis of cyclodextrin-based catalysts. The secondary alcohols of cyclodextrins are relatively unreactive due both to steric hindrance and intramolecular hydrogen bonds between the C2 and C3 hydroxyls, and are therefore unaffected by limited oxidation with DMP.13 The only complication in the oxidation of cyclodextrins by DMP is that a mixture of regioisomers of polyformyl cyclodextrin results. The number of regioisomers can be determined from the equation below, where N is number of glucose molecules in the cyclodextrin, and n is the degree of oxidation. For example, -CD after one degree of oxidation results in one regioisomer, 6Adeoxy-6A-formyl--CD, and after two degrees of oxidation produces three regioisomers, 6A, 6Bdideoxy-6A, 6B-diformyl--CD, 6A, 6C-dideoxy-6A, 6C-diformyl--CD, and 6A, 6D-dideoxy-6A, 6D-diformyl--CD (see Figure 7). 8 Two Degrees of Oxidation One Degree of Oxidation D E C B D E F G 6A-deoxy6A-formyl-CD C Number of Regioisomers N = glucose units n = degree of oxidation D E F G B E F A G C B F G (N-1)! (N-n)!n! 6A, 6A, 6B-dideoxy- 6A, 6C-dideoxy- 6A, 6D-dideoxy6B-diformyl- 6A, 6C-diformyl- 6A, 6D-diformyl-CD -CD -CD Figure 7. Polyformyl cyclodextrin regioisomerism Dicyanoanthracene As a result of its three-ring aromatic -electron system, DCA is one of many molecules capable of photoexcitation and subsequent photoinduced electron transfer oxidation reactions (see Figure 8). For photooxidation to occur, a photosensitive acceptor molecule in solution must be stimulated by electromagnetic radiation (typically in the UV or visible regions), and subsequently enable the abstraction of an electron from a nearby donor molecule. In the present study, the DCA capped -CD acts as the acceptor molecule, and the donor is theoretically the guest molecule that resides in the cavity of the DCA capped -CD. CN CN Figure 8. 9, 10-Dicyanoanthracene (DCA) Abelt and coworkers have previously tethered and capped anthraquinone and benzophenone functionalities to -CD, only to learn that the photoexcited functionalities abstract hydrogen from the cyclodextrin moiety, effectively quenching the host molecule and rendering it incapable of oxidizing the guest through electron transfer.14, 15 In 1994 Abelt and coworkers began working on DCA substituted -CD, since its singlet excited state is much less likely to 9 abstract hydrogen than the triplet excited state of anthraquinone and benzophenone.16 DCA exhibits a relatively low reduction potential (-0.82 V) and is thus capable of oxidizing a variety of chemicals.17 Unfortunately it appeared likely from fluorescence quenching studies that the 6A, 6D/C-(DCA-2, 6-disulfonyl)--CD synthesized by Abelt and coworkers in 1994 would exhibit both complexed (dynamic) and uncomplexed photooxidation, thus reducing the desired stereospecificity of the compound (see Figure 9).18 Quenching CN O2S CN SO2 Quenching Figure 9. The fluorescence quenching of 6A, 6D/C-(DCA-2, 6-disulfonyl)--CD In hopes of maximizing the amount of dynamic quenching, Abelt and coworkers synthesized various DCA substituted -CDs that have not yet been analyzed by fluorescence quenching.19 Recently Abelt and Tan synthesized the same 2, 3-DCA capped -CD described in this research. However, their method provided insufficient quantities of product, thereby preventing fluorescence quenching analysis.20 The Wittig Reaction One of the most convenient ways to introduce a carbon-carbon double bond to a molecule is through nucleophilic addition to a carbonyl functionality by a phosphorus ylide, followed by 10 elimination, as reported by Wittig and Geissler (the Wittig reaction).21 In its general form, a phosphorus ylide consists of a phosphorus atom doubly-bonded to an alkyl derivative. The phosphorus also commonly bears three resonance stabilizing phenyl groups. The reactivity of the ylide derives from the resonance form containing a carbanion and adjacent phosphonium ion as shown (see Figure 10).22 P CHR P CHR Figure 10. Phosphonium ylide resonance structure Preparation of the phosphonium ylide occurs through the reaction of an alkyl halide and triphenylphosphine in the presence of potassium-t-butoxide (KOtBu), followed by abstraction of an -hydrogen with a suitable base (see Figure 11). The base strength required to accomplish hydrogen abstraction depends on the ability of the alkyl derivative to stabilize charge by resonance or inductive effects; less stabilized ylides require a stronger base. A less stabilized ylide is consequently more basic and may react with hydroxyls, water, oxygen, and carbon dioxide.23 Therefore, the Wittig reaction has not found wide use in carbohydrate chemistry due to the reactivity of strongly basic ylides with the relatively acidic hydroxyl groups. Solutions to this problem include the formation of a less basic ylide through resonance or inductive stabilization, or in the past by protection of the hydroxyl groups.24 Ph3P + BrCH2R KOtBu Ph3P CH2R BuLi Br Figure 11. Preparation of the Wittig reagent 11 Ph3P CHR The ylide can be stabilized through the use of conjugating substituents. These substituents, such as carboxyalkanes, nitriles, and sulfones, help stabilize the carbanion through resonance electron withdrawal. Less stabilizing substituents, including phenyls and allyls, provide only semistabilized ylides. With stabilized ylides, the localization of charge on the carbanion becomes reduced, and thus the general reactivity of the ylide is decreased. Although the rate of the Wittig reaction is therefore slightly decreased, the ylide-hydroxyl acid-base side reaction is dramatically reduced.24 The magnitudes of hydroxyl pKa are also important in the reactions of ylides. Hydroxyls with a high pKa react more slowly with ylides. Sufficiently high carbohydrate pKa values can often be obtained by using a slightly polar, protic solvent. However, a strongly protic solvent would inhibit the Wittig reaction through proton-anion interactions, and therefore a slightly polar, relatively aprotic solvent must be used. Common examples include tetrahydrofuran (THF), N,N-dimethylformamide (DMF), DMSO, and pyridine. The hydroxyl groups of cyclodextrins exhibit pKa values on the order of 12 in DMSO and 18 in DMF, thus in these solvents ylides would be unlikely to condense with the hydroxyl moieties.24, 25 Through the use of semistabilized or stabilized ylides and proper solvents, one is able to perform Wittig reactions on unprotected carbohydrates without significant side product formation, and with yields greater than possible when employing conventional methods of hydroxyl protection through acetylation or otherwise. Several examples of the Wittig reaction on unprotected carbohydrates exist.26 Work on D-aldohexoses and their derivatives are most relevant to cyclodextrins. Giannis and Sandhoff have conducted Wittig olefinations of glucosamine derivatives and unprotected D-glucose and D-galactose, as well as related compounds.27, 28 Since cyclodextrins exist as macromolecules of -1,4-glucose chains, the 12 reactions of Giannis and Sandhoff answer many of the questions regarding the analogous reaction with cyclodextrins. Abelt and coworkers have recently used the Wittig reaction with CD successfully.20, 25 Ylide stabilization also effects the stereochemistry of the olefinic bond. As a general rule, nonstabilized ylides tend to yield cis alkenes, and stabilized or semistabilized ylides favor trans alkenes. Many studies have examined the formation of the Wittig intermediates which lead to both cis and trans isomers. 31 P NMR studies of nonstabilized ylides have confirmed the intermediate formation of oxaphosphetane compounds, while the presence of lithium salts, which slow the reaction of nonstabilized ylides, appears to encourage the formation of the trans isomer through phosphorus betaine intermediates.24 Current thinking suggests that for nonstabilized ylides, the isomerism of the olefinic bond is governed by a kinetic mechanism, which results in the cis alkene. However, when the Wittig reaction is sufficiently slowed, passing through the reversible betaine intermediate, thermodynamic mechanisms predominate and typically lead to the formation of the trans isomer (see Figure 12).23 R3 O O PPh3 C CH R3 R1 O PPh3 C CH R3 R1 R2 H R3 R1 C Ylide R2 R2 R2 Betaine Ph3P CH2R1 Oxaphosphetane + O PPh3 Figure 12. General mechanism for the Wittig reaction In 1997, Abelt and coworkers used the Wittig reagent 9, 10-dicyanoanthracenyl-2methylenetriphenylphosphonium, to attach the DCA moiety to 6-deoxy-6-formyl--CD. The olefinic bond of the DCA tethered -CD was shown to be the trans conformation based on NMR studies.25 Tan later demonstrated that the double Wittig product 6A, 6B-dideoxy-6A, 6B-(9,10- 13 dicyanoanthracenyl-2, 3-dimethylene)--CD could be produced from the reaction of the 9, 10dicyanoanthracenyl-2, 3-bis(methylenetriphenylphosphonium) Wittig reagent and the 6A, 6Bdideoxy-6A, 6B-diformyl--CD synthesized via the Nace reaction (see Figure 13).20 NC NC CN CN 6A-Deoxy-6A-(DCA-2-methylene)--CD 6A, 6B-Dideoxy-6A, 6B-(DCA-2, 3-dimethylene)--CD Figure 13. Tethered and capped DCA--CD 14 Experimental -CD from Cerestar was recrystalized twice from water and dried under a vacuum of approximately 0.1 Torr overnight. DMSO was distilled under reduced pressure from calcium hydride in an oil bath heated to 60 ºC, and stored under nitrogen. LRP-2 C-18 bonded silica gel (37-53 m) was used for reversed-phase column chromatography under linear gradient elution. Eight 100 mL solutions of acetonitrile in water were used: 0%, 1%, 2%, 4%, 8%, 10%, 15%, and 20%. A Waters 244 system, equipped with an UV absorption detector (254 nm) and a Whatman Partisil 10 ODS-3 column, was used for all preparative high performance liquid chromatography (HPLC). A gradient elution from 5% to 83% aqueous acetonitrile was applied over one hour at 6.0 mL per minute. Thin layer chromatography (TLC) analyses were conducted on plates of 250 m silica gel HLF (Uniplate™) with organic binder and UV254. A solution of n-butanol, ethanol, and water (5:4:3) was used for the eluant, and products were detected with short wave UV light and subsequent vanillin stain. Proton NMR studies were conducted on a GE QE-300 spectrometer. Dess-Martin Periodinane 2-Iodobenzoic acid (10.18 g, 41.0 mmol) was solvated in 90 mL of 0.73 M aqueous sulfuric acid and heated to 60 ºC. Over a 30-minute period 8.93 g of potassium bromate (53.5 mmol) was added while vigorously stirring. The solution was heated to 68 ºC and allowed to stir overnight. A white compound was collected on a Buchner funnel, washed with 1000 mL of water and twice with 50 mL of ethanol, and added to 80 mL acetic anhydride. The solution was 15 heated to 80 ºC, 0.05 g tosyl alcohol hydrate (0.29 mmol) was added with stirring, and the solution was allowed to stir overnight under a calcium chloride drying tube. The white solid (7.5 g, 17.7 mmol, 43% yield) was again collected on a Buchner funnel and washed with copious amounts of anhydrous ether (5 x 50 mL). Polyformyl -CD Two grams of -CD (1.8 mmol) were dissolved in 50 mL of DMSO under nitrogen. Five portions of 1.5 equivalents of DMP (each 1.5 g, 3.5 mmol) were added with stirring at room temperature over 1.5 hours, 2.5 hours, 7.0 hours, 1.5 hours, and 30 hours. The solution slowly changed color from white to a deep tan. Water (250 mL) was added and the suspension was stirred overnight. The solution was filtered and the water was removed from the filtrate by rotary evaporation. Acetone (500 mL) was added to the residue. After stirring for 2 hours, the suspension was filtered and the filtrate was rotovapped. The DMSO was removed by distillation at 60 ºC under a vacuum of 0.1 Torr. 1H NMR (see Figure 17). 2, 3-Bis(bromomethyl)-9, 10-dicyanoanthracene One gram of 2, 3-dimethyl-9, 10-dicyanoanthracene (3.9 mmol) was dissolved in 500 mL of carbon tetrachloride and heated to reflux at 96 ºC; the synthesis of 2, 3-dimethyl-9, 10dicyanoanthracene is described by Harwell (see Figure 14).29 Bromine (1.3 g, 8.1 mmol) was weighed into 25 mL of carbon tetrachloride and added at an approximate rate of one drop per second with stirring. A halogen floodlight was directed at the reaction flask and the solution was heated to reflux at 96 ºC for 3 additional hours under a calcium chloride drying tube. The majority of the carbon tetrachloride was distilled off, and 1500 mL of methylene chloride was 16 added. A yellow solid was precipitated from the methylene chloride and collected on a Buchner funnel by sequential addition of 200 mL 2% aqueous sodium sulfate, 200 mL 2% aqueous sodium carbonate, and lastly about 1000 mL of water. The yellow solid (1.57 g, 3.8 mmol, and 97% yield) was recrystalized from toluene (200 mL) by dissolving the solid in toluene, hot filtering the solution, and carefully adding ethanol (400 mL) at boiling. (Caution! Since the boiling point of toluene is greater than that of ethanol, rapid addition of large volumes of ethanol may cause intense vaporization.) 1H NMR (see Figure 18). CH 3 CH3 CH 3 HO CH 3 CH 3 O Al(s) HgCl2 O CH 3 H3 C O Br Br2 Br CH3 N CH3 CuCN N2 CH 3 CH 3 CH 2Br CH 2Br Br2 CCl4 NC CN NC CN CaCl2 Figure 14. The synthesis of 9, 10-dicyano-2, 3-bis(bromomethyl)anthracene 9, 10-Dicyanoanthracenyl-2, 3-bis(methyltriphenylphosphonium bromide) 2, 3-Bis(bromomethyl)-9, 10-dicyanoanthracene (0.22 g, 0.53 mmol) was dissolved in 80 mL of benzene under nitrogen. Triphenylphosphine (0.28 g, 1.07 mmol) was added and the solution was heated to reflux at 110 ºC overnight with stirring. A yellow solid (0.11 g, 0.16 mmol, and 31% yield) was collected on a Buchner funnel and dried under a vacuum of 0.1 Torr. 17 Polyformyl-6A, 6B-dideoxy-6A, 6B-(9,10-dicyanoanthracenyl-2, 3-dimethylene)--CD Polyformyl--CD (0.40 g, 0.36 mmol) was dissolved in 40 mL of DMSO, 40 mL of benzene, and heated to reflux at 190 ºC under nitrogen. Unfortunately, the condenser was absent, the solvent was boiled off, and the polyformyl--CD was slightly charred. Another 40 mL of DMSO and 40 mL of benzene was added, the polyformyl--CD was solvated as much as possible, and the solution was heated to reflux at 190 ºC under nitrogen in a Dean-Stark trap to collect water. In a separate flask at room temperature, 0.11 g of 9, 10-dicyanoanthracenyl-2, 3bis(methyltriphenylphosphonium bromide) (0.16 mmol) was dissolved in 30 mL of DMSO, yielding a yellow solution. Potassium-t-butoxide (0.04 g, 0.36 mmol) was added with stirring under nitrogen. After 10 minutes a deep green color was achieved. The solutions were mixed and heated overnight to 70 ºC under nitrogen. The benzene was removed by rotovap and the DMSO was distilled off at 60 ºC under a vacuum of 0.1 Torr. Acetone (200 mL) was added to the flask to precipitate a black solid, which was collected on a Buchner funnel. Product was thought to be present according to TLC. The product was purified using reversed-phase column chromatography and HPLC and showed a retention time of 6.66 minutes. 1H NMR did not show presence of the desired compound. 18 Results and Discussion The synthesis of 6A, 6B-dideoxy-6A, 6B-(9,10-dicyanoanthracenyl-2, 3-dimethylene)-CD is divided into four main steps (only three of which were attempted). First, the -CD needs to be oxidized to polyformyl--CD by reaction with DMP to serve as an appropriate precursor for the Wittig reaction. The second step is the synthesis of the Wittig capping reagent, 9, 10dicyanoanthracenyl-2, 3-bis(methyltriphenylphosphonium bromide). These two reagents are then combined according to the Wittig reaction to produce polyformyl-6A, 6B-dideoxy-6A, 6B(9,10-dicyanoanthracenyl-2, 3-dimethylene)--CD. A final step is necessary to remove additional formyl groups from the primary face of the -CD. In the first segment of this synthesis, DMP was produced according to established procedures.8, 9 However, the yield was much lower than reported in the literature (43% compared to 85%), probably due the increase in reaction temperature (55 ºC vs. 60 ºC) and time (3.6 hours vs. overnight), previously postulated as sources of decreased yield.9 Utilization of a Dean-Stark trap, to remove any water before and during oxidation of -CD with the very hygroscopic DMP, might also have improved the yield. The oxidation of -CD to 6A-formyl-CD requires 1.5 equivalents of DMP to reach completion, and can be rationalized nonstoichiometrically according to the mechanism below (see Figure 15). Even though five degrees of DMP (7.5 eq) is theorized to completely oxidize the -CD at five of the seven glucose units, the 1H NMR spectrum was not convincing (see Figure 17).13 19 O O AcO OH I + OAc I O H H C H H H2O O O ---- H H O AcO I O O + C C O O Figure 15. The oxidation of -CD using 1.5 equivalents of DMP Furthermore, the Wittig capping agent is subject to strict bond restraints, which limit the cap to A, B substitution. After multiple degrees of oxidation of -CD by DMP, only a portion of the -CD will exhibit the required A, B-diformyl construction, and isolated formyl groups (without an adjacent formyl group) will allow tethering but forbid capping. Cornwell et al. suggest that multiple oxidations occur sequentially (as opposed to simultaneously) and without regiochemical influence from the preexisting formyl groups.13 If we assume that oxidation is complete, then the probability of finding a polyformyl--CD with at least two adjacent formyl groups can be readily calculated to be 33% for two degrees of oxidation (see Figure 7), 60% for three degrees of oxidation, 80% for four degrees of oxidation, 93 % for five degrees of oxidation, and 100% for six or seven degrees of oxidation. Therefore, the Wittig reaction with polyformyl-CD leads to a small decrease in yield as a result of the fact that a maximum of 93% can be A, B-capped. The synthesis of the Wittig capping agent, 9, 10-dicyanoanthracenyl-2, 3bis(methyltriphenylphosphonium bromide), was based on the original construction by Wittig and Geissler.21 However, in order to create a bis-phosphonium ylide, the quantity of triphenylphosphine was doubled. The mechanism is outlined below; the phosphorus lone pair of 20 the triphenylphosphine attacks the brominated carbons of 2, 3-bis(bromomethyl)-9, 10dicyanoanthracene to produce the Wittig bis-phosphonium salt, and tert-butoxide in solution abstracts an -hydrogen to produce the ylide (see Figure 16). CN CN Br Br P Br PPh3 PPh3 CN CN Br P H CN PPh3 O-But Br CN O-But CN CN H PPh3 PPh3 PPh3 PPh3 CN CN Figure 16. Synthesis of the bis-phosphonium ylide The Wittig reaction of the polyformyl--CD and the bis-phosphonium ylide proceeds via the general mechanism developed by Wittig and Geissler (see Figure 12).21 The Wittig capping reagent initially creates an olefinic tether to one molecule of polyformyl--CD. Since the Wittig capping reagent is a bis-phosphonium ylide, however, it may react with a second formyl group. Due to bond constraints, the second formyl group must be on the adjacent glucose unit, thus yielding an A, B-capped--CD. The HPLC retention time of the product isolated in this work was significantly different than that expected based on the work of Tan. Furthermore, the A, Bcapped--CD product was not visible by 1H NMR, suggesting that the slight charring of the polyformyl--CD that occurred may have prevented the Wittig reaction from proceeding as expected.23 21 Completing the synthesis of the A, B-capped-b-CD would have required reduction of the extra formyl groups remaining after the Wittig reaction. Since the Wittig product could not be sufficiently identified or isolated, reduction was not possible. However, according to the literature, reaction with sodium borohydride would sufficiently reduce all formyl groups back to their corresponding hydroxyls without reacting with other functional groups present on the molecule.30 This method may permit production of the desired 6A, 6B-dideoxy-6A, 6B-(9,10dicyanoanthracenyl-2, 3-dimethylene)--cyclodextrin in the future. 22 Conclusion Mistakes in the research method prevented the isolation and positive identification of the desired 6A, 6B-dideoxy-6A, 6B-(9,10-dicyanoanthracenyl-2, 3-dimethylene)--CD. However, the synthesis seems to be far simpler than the previous synthesis developed by Tan, which utilized the application of a 4,6-dimethoxybenzene-1, 3-disulfonyl chloride cap followed by removal according to the Nace reaction in order to produce A, B-diformyl--CD.20 The majority of the steps in this reaction are capable of providing product in high yield. However, the Wittig reaction of the bis(phosphonium) ylide consistently results in relatively poor yield, probably due to steric hindrance and bond restraints, and must be greatly scaled up. The possibility exists that the -CD oxidation step and Wittig capping could be combined using a one-pot method according to Barrett et al., thus permitting a faster, more efficient synthetic route.31 Finally, fluorescence quenching studies need to be conducted to determine the ability of the product molecule to act as a photocatalytic host. 23 Figure 17. 1H NMR spectrum of polyformyl--CD 24 Figure 18. 1H NMR spectrum of 2, 3-bis(bromomethyl)-9, 10-dicyanoanthracene 25 References 1 Parker, S. P., Editor-in-Chief. McGraw-Hill Encyclopedia of Chemistry: 2nd Ed. McGrawHill, Inc.: New York, 1992. 2 Bender, M. L.; Komiyama, M. Cyclodextrin Chemistry. Springer-Verlag: New York, 1978. 3 Szejtli, J., Ed. Proceedings of the First International Symposium on Cyclodextrins. D. Reidel Publishing Co.: Boston, 1982. 4 Breslow, R.; Zhang, B. J. Am. Chem. Soc. 1994, 116, 7893-7894. 5 Neckers, D. C.; Paczkowski, J. J. Am. Chem. Soc. 1986, 108, 291-292. 6 Ye, H.; Tong, W.; D’Souza, V. T. J. Am. Chem. Soc. 1992, 114, 5470-5472. 7 Kuroda, Y.; Ito, M.; Sera, T.; Ogoshi, H. J. Am. Chem. Soc. 1993, 115, 7003-7004. 8 Dess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1991, 113, 7277-7287. 9 Ireland, R. E.; Liu, L. J. Org. Chem. 1993, 58, 2899. 10 Hanson, G. J.; Lindberg, T. J. Org. Chem. 1985, 50(25), 5399-5401. 11 Danishefsky, S. J.; Mantlo, N. B.; Yamashita, D. S.; Schulte, G. J. Am. Chem. Soc. 1988, 110, 6890-6891. 12 Robins, M. J.; Samano, V.; Johnson, M. D. J. Org. Chem. 1990, 55, 410-412. 13 Cornwell, M. J.; Huff, J. B.; Bieniarz, C. Tetrahedron Lett. 1995, 36(46), 8371-8374. 14 Aquino, A. M.; Abelt, C. J.; Berger, K. L.; Darragh, C. M.; Kelley, S. E.; Cossette, M. V. J. Am. Chem. Soc. 1990, 112, 5819-5824. 15 Berger, K. L.; Nemecek, A. L.; Abelt, C. J. J. Org. Chem. 1991, 56, 3514-3520. 16 Acquavella, M. F.; Evans, M. E.; Farraher, S. W.; Névoret, C. J.; Abelt, C. J. J. Org. Chem. 1994, 59, 2894-2897. 17 Chandross, E.; Ferguson, J. J. Chem. Phys. 1967, 47, 2557-2560. 26 18 Acquavella, M. F.; Evans, M. E.; Farraher, S. W.; Névoret, C. J.; Abelt, C. J. J. Chem. Soc., Perkin Trans. 2. 1995, 59, 385-388. 19 Hubbard, B. K.; Beilstein, L. A.; Heath, C. E.; Abelt, C. J. J. Chem. Soc., Perkin Trans. 2. 1996, 1005-1009. 20 Tan, J. The Synthesis of 6A, 6B-Dideoxy-6A, 6B-(9,10-Dicyanoanthracenyl-2, 3-Dimethylene)- -Cyclodextrin. Master’s thesis at the College of William and Mary. 1997. 21 Wittig, G.; Geissler, G. Annalen. 1953, 580, 44. 22 Vollhardt, K. P. C.; Schore, N. E. Organic Chemistry. Freeman and Company: New York, 1994. 23 Sutherland, I. O., Ed. Comprehensive Organic Chemistry: The Synthesis and Reactions of Organic Compounds, vol. 2. Pergamon Press: New York, 1979. 24 Maryanoff, B.E.; Reitz, A. B. Chem. Rev. 1989, 89, 863-927. 25 Ivy, R. L.; Hafez, A. M.; Abelt, C. J. J. Org. Chem. 1997, 62, 6415-6416. 26 Horton, D.; Koh, D., Carbohydrate Res. 1993, 250, 231-247. 27 Giannis, A.; Münster, P.; Sandhoff, K.; Steglich, W. Tetrahedron. 1988, 44, 7177-7180. 28 Henk, T.; Giannis, A.; Sandhoff, K. Liebigs Ann. Chem. 1992, 167-168. 29 Harwell, A. The Synthesis of 9, 10-Dicyanoanthracene-2, 3-Dicarboxylic Acid. Undergraduate honor’s thesis at the College of William and Mary. 1996. 30 Paquette, L. A., Editor-in-Chief. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons: New York, 1995. 31 Barrett, A. G. M.; Hamprecht, D.; Ohkubo, M. J. Org. Chem. 1997, 62, 9376-9378. 27 Vita Michael Anthony Repucci Born the oldest of three sons to Michael Joseph Repucci and Janet Ruth Knorr Repucci on July 9th, 1976 in Bethesda, Maryland. Graduated from Hopkinton High School in June of 1994 in Hopkinton, Massachusetts. Enrolled in the Bachelor of Science program in chemistry and psychology at The College of William and Mary in August of 1994. After completing his degree the author will continue his studies, at Cornell University Graduate School of Medical Sciences, in the field of neuroscience and neuropharmacology. 28