Grade 12 Chemistry (SCH4U) Course Syllabus
advertisement

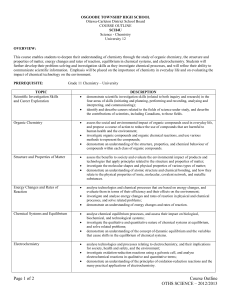
Course: Chemistry,Grade 12 University Preparation Course Code: SCH4U PREREQUISITE: Chemistry, Grade 11, University Preparation (SCH3U). Students should have completed MPM2D and recommended that they have taken Physics 3U. Textbook: McGraw-Hill Ryerson Chemistry 12. Teacher: Rola Wansa Email: rola.wansa@ocdsb.ca Course website: http://rolasch4u.wikispaces.com/ Office: room 509 SUPPLIES: You will need the following supplies to do work in this course: 1. 3 – ring binder with loose leaf paper. 2. Pencils, eraser, ruler and calculator. A pen is needed for formal lab reports. 3. A non-graphing calculator with a logarithmic function. 4. You may borrow a textbook. You are responsible for the full cost if the text is lost or damaged. COURSE DESCRIPTION: This course enables students to deepen their understanding of chemistry through the study of organic chemistry, the structure and properties of matter, energy changes and rates of reaction, equilibrium in chemical systems, and electrochemistry. Students will further develop their problem-solving and investigation skills as they investigate chemical processes, and will refine their ability to communicate scientific information. Also students will evaluate the importance of chemistry in everyday life and the impact of chemical technology on the environment. Big Ideas: Unit (textbook order) Chemistry focus for Learning Expectations 1. Organic Chemistry ·Organic compounds have predictable chemical and physical properties determined by their respective structures. · Organic chemical reactions and their applications have significant implications for society, human health, and the environment · The nature of the attractive forces that exist between particles in a substance determines the properties and limits the uses of that substance. · Technological devices that are based on the principles of atomic and molecular structures can have societal benefits and costs. · Energy changes and rates of chemical reactions can be described quantitatively. · Efficiency of chemical reactions can be improved by applying optimal conditions. · Technologies that transform energy can have societal and environmental costs and benefits. · Chemical systems are dynamic and respond to changing conditions in predictable ways. · Applications of chemical systems at equilibrium have significant implications for nature and industry. 2. Structure and Properties of Matter 3. Energy Changes & Rates of Reaction 4. Chemical Systems & Equilibrium 5. Electrochemistry . Oxidation and reduction are paired chemical reactions in which electrons are transferred from one substance to another in a predictable way. · The control and applications of oxidation and reduction reactions have significant implications for industry, health and safety, and the environment. Laboratory Safety: Each student must have received safety instruction and signed a contract relating to lab safety before carrying out any of the lab activities in this course All laboratory resources and materials will be supplied by the school. EVALUATION: The achievement chart identifies four levels, based on achievement of the overall expectations: Level 1 achievement falls below the provincial standard (50-59%) Level 2 achievement approaches the provincial standard (60-69%) Level 3 achievement is at the provincial standard (70-79%) Level 4 achievement surpasses the provincial standard (80-100%) The report card grade will be based on evidence of student performance which might include observations, conversations and student products. Consideration will be given to more recent evidence (skill development) and the most consistent level of achievement. Term Work (70%) will include a variety of assessment tasks designed to demonstrate students’ development in their knowledge and understanding, thinking and inquiry, communication and application, of all overall expectations. Summative evaluation (30%) takes place towards the end of the semester, is completed in class, and provides the final opportunity for students to demonstrate what they know, and the skills they have learned, based on the overall expectations. NOTES: 1. 2. 3. 4. You are required to do all the assigned work, including tests. There are no make up tests given because there will be a multiple opportunity available to show the evidence of learning of the overall expectations. If you know you will miss a test or deadline then discuss the situation with your teacher before the date. If you are forced to miss a test or deadline, email the teacher about it to justify the absence. You must write a final examination. Your credit will be in jeopardy if a final exam is not written. Good attendance is required. Too many absences can result in the loss of a credit and withdrawal from the school. Please review the School’s policy on Assessment and Evaluation and other policies found in the Student Planner. STUDY TIPS: Access the teacher website for this course to find solutions for homework questions and additional lessons to help you understand certain topics. Keep up your homework assignments daily. Homework helps to reinforce the concepts you study in class. Even if you are very busy, spend a little time every day. Spend on average an hour daily on your chemistry homework. Try to do extra exercises. Practice improves your speed, accuracy and recall. Overall Expectations: SCH4U A. Scientific Investigation Skills and Career Exploration Throughout this course, students will: demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. B. Organic Chemistry By the end of this course, students will: assess the social and environmental impact of organic compounds used in everyday life, and propose a course of action to reduce the use of compounds that are harmful to human health and the environment; investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds; demonstrate an understanding of the structure, properties, and chemical behaviour of compounds within each class of organic compounds. C. Structure and Properties of Matter By the end of this course, students will: assess the benefits to society and evaluate the environmental impact of products and technologies that apply principles related to the structure and properties of matter; investigate the molecular shapes and physical properties of various types of matter; demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. D. Energy Changes and Rates of Reactions By the end of this course, students will: analyse technologies and chemical processes that are based on energy changes, and evaluate them in terms of their efficiency and their effects on the environment; investigate and analyse energy changes and rates of reaction in physical and chemical processes,and solve related problems; demonstrate an understanding of energy changes and rates of reaction. E. Chemical Systems and Equilibrium By the end of this course, students will: analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. F. Electrochemistry By the end of this course, students will: analyse technologies and processes relating to electrochemistry, and their implications for society, health and safety, and the environment; investigate oxidation-reduction reactions using a galvanic cell, and analyse electrochemical reactions in qualitative and quantitative terms; demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry.