Notre Dame Catholic Secondary School Course Code: SCH4U1
advertisement

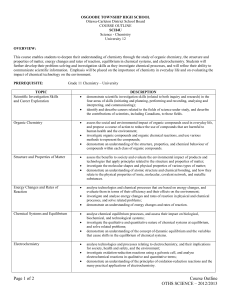
SCIENCE DEPARTMENT Notre Dame Catholic Secondary School Course Code: SCH4U1 Course Name: Grade 12 Chemistry Level: University Instructor: Mr. Wynhofen Period: ONE Room Number: 241 Course Overview: This course enables students to deepen their understanding of chemistry through the study of organic chemistry, the structure and properties of matter, energy changes and rates of reaction, equilibrium in chemical systems, and electrochemistry. Students will further develop their problem-solving and investigation skills as they investigate chemical processes, and will refine their ability to communicate scientific information. Emphasis will be placed on the importance of chemistry in everyday life and on evaluating the impact of chemical technology on the environment so that students to learn to be reflective, critical, and creative thinkers, as well as discerning believers. They can then make appropriate decisions in light of Gospel values and Church teachings. Through labs and experimentation, students learn to be collaborative contributors to an interdependent team, respecting the rights, responsibilities, and contributions of others. Specific Strands of Study and Overall Expectations include: A. Scientific Investigation Skills and Career Exploration [Incorporated throughout course.] A1. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analyzing and interpreting, and communicating); A2. identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. B. Organic Chemistry [Chapters 1/2 ~ 12 classes] B1. assess the social and environmental impact of organic compounds used in everyday life, and propose a course of action to reduce the use of compounds that are harmful to human health and the environment; B2. investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds; B3. demonstrate an understanding of the structure, properties, and chemical behaviour of compounds within each class of organic compounds. C. Structure and Properties of Matter [a) Ch. 3 - Atomic Theories (~9 classes) ; b) Ch. 4 - Chemical Bonding (~9 classes)] C1. assess the benefits to society and evaluate the environmental impact of products and technologies that apply principles related to the structure and properties of matter; C2. investigate the molecular shapes and physical properties of various types of matter; C3. demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. D. Energy Changes and Rates of Reaction [a) Ch. 5 - Thermochemistry [(~8 classes) ; b) Ch. 6 - Kinetics (~4 classes)] D1. analyse technologies and chemical processes that are based on energy changes, and evaluate them in terms of their efficiency and their effects on the environment; D2. investigate and analyse energy changes and rates of reaction in physical and chemical processes, and solve related problems; D3.demonstrate an understanding of energy changes and rates of reaction. E. Chem. Systems and Equilibrium[a) Ch. 7 – Chem. Systems and Equ’m [(~12 classes) ; b) Ch. 8 - Acid-Base Equ’m (~12 classes)] E1. analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; E2. investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; E3. demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. F. Electrochemistry [Chap. 9 ~ 9 classes] F1. analyse technologies and processes relating to electrochemistry, and their implications for society, health and safety, and the environment; F2. investigate oxidation-reduction reactions using a galvanic cell, and analyse electrochemical reactions in qualitative and quantitative terms; F3. demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry. Efforts will be made to meet the individual learning needs of students. Resources: Evaluation Efforts willBreakdown: be made to meet the individual learning needs of students. [TERM = 70% ; EXAM = 30%] [K = Knowledge ; I = Investigation ; C = Communication; A = Application] % Overall 1. Tests (7) a) Knowledge (20% of term) -----------------------------------------14.0 b) Investigation (20% of term)----------------------------------------14.0 c) Communication (10% of term) -------------------------------------7.0 d) Application (10% of term) ------------------------------------------7.0 STSE 2. Labs & Assignments a) Investigation (15% of term) ---------------------------------------10.5 b) Knowledge (5% of term) --------------------------------------------3.5 b) Communication (5% of term)---------------------------------------3.5 3. STSE Portfolio a) Application (15% of term)-----------------------------------------10.5 4. Final Exam -----------------------------------------------------------30 [Exam Breakdown: K= 25%; I = 35%; C = 15%; A = 25%] The course will use a variety of resources including video, CD-ROM, Internet Applications and a variety of print sources. The textbook, Nelson Chemistry 12, will be distributed to students during the first week of the course. The text and all other resources assigned to students are the responsibility of the student. Any damage incurred will result in payment for replacement. Replacement cost for the text is $104. Evaluation Structure:: Knowledge/Understanding Thinking/Investigation Communication Application 25% 35% 15% 25% The above is reflected both in the term work (70% of the final mark) and the summative work (30% of the final mark). Summative work consists of the Final Exam only. Evaluation Policy Students will be assessed & evaluated according to the work produced & skills displayed. Methods of providing feedback will include assessing work in process & evaluating completed assignments, tests, co-operative learning activities, simulations and presentations. Peer & self-evaluations will also be utilized. Student marks will be determined by evaluating process & product according to 4 categories & 4 levels. Please see the chart below for specific skills and key words used to determine student competency in the different categories. Level Category Knowledge/Understanding Knowledge of facts & terms Understanding of concepts & relationships Thinking/Investigation Critical thinking skills Creative thinking skills Inquiry Skills Communication Communication of ideas and information Use of symbols & visuals Oral & written communication Level 1: 50-59% Level 2: 60-69% Level 3: 70-79% Level 4: 80-100% -Limited display of knowledge, skills and ability to apply concepts -Some success in displaying knowledge, skills and application of concepts -Considerable display of knowledge skills and ability to apply concepts -Thorough understanding of concepts and ability to communicate, think creatively and apply concepts Application Applications in familiar contexts Transfer of concepts to new contexts Making logical conclusions and predictions Use of technology Making connections Feedback will also be provided for student learning skills. Skills like working independently, team work, organization, work habits and homework, and initiative are assessed independently student achievement and will be conducted through the use of a rubric indicating specific criteria to be achieved to receive each of the following letter grades: E –Excellent G – Good S – Satisfactory N - Needs Improvement MISSELANEOUS ITEMS: * LATE ASSIGNMENTS: Assignments submitted after the Primary Due Date established by the teacher will be accepted with a penalty of 5% off for the first day late, 10% off for the second day late, and zero for subsequent days. MISSED TESTS: DON’T BE ABSENT FOR TESTS!...if unavoidable: *Missed test #1 Student will write on the day he/she returns to class…with acceptable excuse (e.g. Funeral, serious illness, surgery etc.) and note from parent/guardian.) *Missed test #2 Student will not be permitted to write test - weight of test will be added to final exam. *Missed test #3 = ZERO!! **Regardless of what the excuse may be!! PLAGIARISM in ANY FORM reflects academic dishonesty and will result in a mark of zero for the assignment, and a referral to the student’s Vice Principal. All citations and bibliographies should conform to MLA style. ATTENDANCE/PUNCTUALITY: To be successful in this class, coming to class daily and on time is ESSENTIAL!! (At 5 lates an assignment will be given. Subsequent lates will result in parental contact and VP intervention. UNIFORM Student’s are expected to be in full uniform at all times!! Noncompliance will result in VP intervention. PED’s Not permitted in the school. This is a Board policy. FOOD No food in class. Water is permitted.