IONA CATHOLIC SECONDARY SCHOOL Science Department Course Code: SCH4U1
advertisement

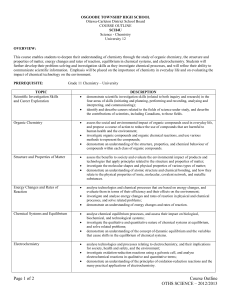
Science Department IONA CATHOLIC SECONDARY SCHOOL Course Code: SCH4U1 Course Name: Chemistry, University Preparation, Grade 12 This course enables students to deepen their understanding of chemistry through the study of organic chemistry, the structure and properties of matter, energy changes and rates of reaction, equilibrium in chemical systems, and electrochemistry. Students will further develop their problem-solving and investigation skills as they investigate chemical processes, and will refine ability toExpectations communicate scientific information. Emphasis will be placed on Strands of Study andtheir Overall include: the importance of chemistry in everyday life and on evaluating the impact of chemical technology on the environment. Prerequisite: SCH3U1 Strands of Study and Overall Expectations include: A1. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); A2. identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. B1. assess the social and environmental impact of organic compounds used in everyday life, and propose a course of action to reduce the use of compounds that are harmful to human health and the environment; B2. investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds; B3. demonstrate an understanding of the structure, properties, and chemical behaviour of compounds within each class of organic compounds. C1. assess the benefits to society and evaluate the environmental impact of products and technologies that apply principles related to the structure and properties of matter; C2. investigate the molecular shapes and physical properties of various types of matter; C3. demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. D1. analyse technologies and chemical processes that are based on energy changes, and evaluate them in terms of their efficiency and their effects on the environment; D2. investigate and analyse energy changes and rates of reaction in physical and chemical processes, and solve related problems; D3. demonstrate an understanding of energy changes and rates of reaction. E1. analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; E2. investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; E3. demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. F1. analyse technologies and processes relating to electrochemistry, and their implications for society, health and safety, and the environment; F2. investigate oxidation-reduction reactions using a galvanic cell, and analyse electrochemical reactions in qualitative and quantitative terms; F3. demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry. Efforts will be made to meet the individual learning needs of students in order to ensure these expectations are being met. Course Breakdown UNITS OF STUDY: TEXTS AND RESOURCES: A. Scientific Investigation Skills Chemistry 12, Nelson and Career Exploration B Organic Chemistry C. Structure and Properties of Matter D. Energy Changes and Rates of Reaction E. Chemical Systems and Equilibrium F. Electrochemistry Resources: The course will use a variety of resources including video, CDROM, Internet Applications and a variety of print sources. The textbook will be distributed to students during the first week of the course. The text and all other resources assigned to students are the responsibility of the student. Any damage incurred will result in payment for replacement. Replacement cost for the text will be posted in the classroom. Evaluation Structure:: Knowledge/Understanding Thinking Communication Application 25% 35% 15% 25% The above is reflected both in the term work (worth 70% of the final mark) and the summative work (worth 30% of the final mark). Summative work consists of the Final Exam ( 30%) and a Culminating Activity ( 0 %). Evaluation Policy Students will be assessed & evaluated according to the work produced & skills displayed. Methods of providing feedback will include assessing work in process & evaluating completed assignments, tests, co-operative learning activities, simulations and presentations. Peer & self-evaluations will also be utilized. Student marks will be determined by evaluating process & product according to 4 categories & 4 levels. Please see the chart below for specific skills and key words used to determine student competency in the different categories. Category Knowledge/Understanding: Knowledge of content and understanding of content. Level 1: Level 2: Level 3: Level 4: 50-59% 60-69% 70-79% 80-100% Limited display of: knowledge of content Some success in: knowledge of content Considerable display of: knowledge of content Thorough understanding of: knowledge of content uses creative thinking initiating, processing and planning skills and strategies with limited effectiveness uses creative thinking initiating, processing and planning skills and strategies with some effectiveness uses creative thinking initiating, processing and planning skills and strategies with considerable effectiveness uses creative thinking initiating, processing and planning skills and strategies with a high degree of effectiveness Communication: Expression and organization of ideas and information and use of conventions vocabulary, and terminology of the discipline in oral, graphic, and written forms. communicates, uses conventions and terminology, organizes ideas and information with limited effectiveness communicates, uses conventions and terminology, organizes ideas and information with some effectiveness communicates, uses conventions and terminology, organizes ideas and information with considerable effectiveness communicates, uses conventions and terminology, organizes ideas and information with a high degree of effectiveness Application: Application and transfer of knowledge and skills; Making connections between science, technology, society, and the environment. transfers and applies knowledge and skills to unfamiliar contexts and proposes courses of practical action with limited effectiveness transfers and applies knowledge and skills to unfamiliar contexts and proposes courses of practical action with some effectiveness transfers and applies knowledge and skills to unfamiliar contexts and proposes courses of practical action with considerable effectiveness Thinking: Use of planning skills, processing skills and critical/creative thinking skills. transfers and applies knowledge and skills to unfamiliar contexts and proposes courses of practical action with a high degree of effectiveness Feedback will also be provided for student learning skills. Skills like RESPONSIBILITY, ORGANIZATION, INDEPENDENT WORK, COLLABORATION, INITIATIVE, and SELF-REGULATION are assessed independently from student achievement and will be conducted through the use of a rubric indicating specific criteria to be achieved to receive each of the following letter grades: E –Excellent G – Good S – Satisfactory N - Needs Improvement Other Evaluation Issues LATE ASSIGNMENTS The due dates for major assignments will be clearly articulated by the teacher when the task is assigned. The teacher will establish a deadline and each day late after this date an appropriate penalty will be established. The exact conditions and penalties can be obtained from the school handbook. INCOMPLETE ASSSIGNMENTS Assignments will be graded according to the extent with which they meet the criteria established in the rubric or evaluation structure. MISSED TESTS . Teachers will give the class ample notice for up-coming tests/evaluations. It is the responsibility of the student to make arrangements for an alternative assessment date (which may not be during class time) with the teacher before the scheduled time for the test/evaluation. If a test is missed due to a legitimate reason, verification (note only to be handed in on the day of the student’s return) from a parent/guardian must be given to the subject teacher indicating that the parents are aware the student has missed a test. If a test is missed as a result of truancy, a mark of zero will be assigned with no opportunity for a re-write. PLAGIARISM in any form reflects academic dishonesty and will result in a mark that is determined by the administration in collaboration with the classroom teacher. Further information can be obtained from the school handbook. ATTENDANCE It is the responsibility of each student to be punctual and in attendance, with proper materials, at all classes and scheduled activities. Students who miss classes may put their credit in jeopardy. It is the student’s responsibility to catch up on missed work when absent.