CHUnit 9 with 5E and LEP- FINAL (6-26-08)
advertisement

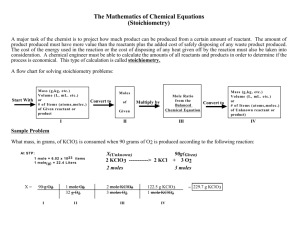
I. Grade Level/Unit Number: 9-12 Unit 9 II: Unit Title: Stoichiometry III. Unit Length: 5 days (on a 90 min. per day block schedule) IV. Major Learning Outcomes: Students should be able to: Interpret coefficients of a balanced equation as mole ratios. Use mole ratios from the balanced equation to calculate the quantity of one substance in a reaction given the quantity of another substance in the reaction. (given moles, particles, mass, or volume and ending with moles, particles, mass, or volume of the desired substance) V. Content Objectives Includes (with RBT Tags): Objective Objective Number 3.03 Calculate quantitative relationships in chemical reactions (stoichiometry): Moles of each species in a reaction. Mass of each species in a reaction. Volumes of gaseous species in a reaction. 3.03-1 H Evaluate reactions to determine limiting reactant and percent yield. RBT Tag C3 VI. English Language Development Objectives (ELD) Included: NC English Language Proficiency (ELP) Standard 4 (2008) for Limited English Proficiency Students (LEP)- English Language learners communicate information, ideas, and concepts necessary for academic success in the content area of science. Suggestions for modified instruction and scaffolding for LEP students and/or students who need additional support are embedded in the unit plan and/or are added at the end of the corresponding section of the lessons. The amount of scaffolding needed will depend on the level of English proficiency of each LEP student. Therefore, novice level students will need more support with the language needed to understand and demonstrate the acquisition of concepts than intermediate or advanced students. VII. Materials/Equipment Needed Activity The Stoichiometry of S’mores Chemistry- Unit 9 Materials Plastic knives, forks & spoons(minimum of 24 each) DRAFT 1 Plastic zip-top bags Hershey’s Chocolate bars 2 Marshmallows (large) Graham crackers Paper plates Napkins Electronic balance(s) Mass and Mole Relationships in a Chemical Reaction safety goggles lab aprons 100-mL beakers solid NaHCO3 plastic pipettes 1M HCl hot plates or Bunsen burners VIII. Detailed Content Description: Please see the detailed content description for each objective in the chemistry support document. The link to this downloadable document is in the Chemistry Standard Course of Study at: http://www.ncpublicschools.org/curriculum/science/scos/2004/24chemistry IX. Unit Notes: This unit is focused on the quantitative relationships among reactant and/or products in a chemical reaction. More specifically, students should be able to interpret coefficients from a balanced equation as mole ratios. Students will learn how to perform mole-mole, mass-mole, mole-mass, and mass-mass stoichiometric calculations. More specifically, students should be able to: Interpret coefficients of a balanced equation as mole ratios. Use mole ratios from the balanced equation to calculate the quantity of one substance in a reaction given the quantity of another substance in the reaction. (given moles, particles, mass, or volume and ending with moles, particles, mass, or volume of the desired substance). In each unit, Goal 1 objectives which relate to the process of scientific investigation are included. In each of the units, students will be practicing the processes of science: observing, hypothesizing, collecting data, analyzing, and concluding. The Goal 1 Objectives are as follows: COMPETENCY GOAL 1: The learner will develop abilities necessary to do and understand scientific inquiry. Chemistry- Unit 9 DRAFT 2 1.01 Design, conduct and analyze investigations to answer questions related to chemistry. Identify questions and suggest hypotheses. Identify variables. Use a control when appropriate. Select and use appropriate measurement tools. Collect and organize data in tables, charts and graphs. Analyze and interpret data. Explain observations. Make inferences and predictions. Explain the relationship between evidence and explanation. Identify how scientists share findings. This goal and these objectives are an integral part of each of the other goals. In order to measure and investigate scientific phenomena, students must be given the opportunity to design and conduct their own investigations in a safe laboratory. The students should use questions and models to formulate the relationship identified in their investigations and then report and share those finding with others Students will be able to: Identify questions and suggest hypotheses. Identify variables. Use a control when appropriate. Select and use appropriate measurement tools. Collect and organize data in tables, charts and graphs. Analyze and interpret data. Explain observations. Make inferences and predictions. Use questions and models to determine the relationships between variables in investigations. Identify how scientists share findings. If a teacher follows this curriculum (s)he will have addressed the goals and objectives of the SCOS. However, teachers may want to substitute other activities that teach the same concept. The unit length has extra time built in for quizzes, going over homework, additional practice depending on the nature of the class, and assessment. Teachers should utilize the textbook as a resource by assigning homework each day and providing additional guided and independent practice. Reference Tables: The North Carolina Chemistry Reference Tables were developed to provide essential information that should be used on a regular basis by students, therefore eliminating the need for memorization. It is suggested that a copy be provided to each student on the first day of instruction. A copy of the reference tables can be downloaded at the following URL: Chemistry- Unit 9 DRAFT 3 http://www.ncpublicschools.org/docs/curriculum/science/scos/2004/chemistry/referencet ables.pdf Essential Questions: Essential questions for this unit are embedded within the unit. Essential questions are those questions that lead to student understanding. Students should be able to answer these questions at the end of an activity. Teachers are advised to put these questions up in a prominent place in the classroom. The questions can be answered in a journal format as a closure. Safety: Students should wear chemical splash goggles during any lab activity involving chemicals. This includes household substances. It is extremely important for the safety and success of your students that you do ALL activities and labs prior to assigning them to students. At the beginning of each lab, the teacher should address any specific safety concerns relating to the activity. Computer Based Activities: Several of the recommended activities are computer based and require students to visit various internet sites and view animations of various biological processes. These animations require various players and plug-ins which may or may not already be installed on your computers. Additionally some districts have firewalls that block downloading these types of files. Before assigning these activities to students it is essential for the teacher to try them on the computers that the students will use and to consult with the technology or media specialist if there are issues. These animations also have sound. Teachers may wish to provide headphones if possible. X. Global Content: Aligned with 21st Skills One of the goals of the unit plans is to provide strategies that will enable educators to develop the 21st Century skills for their students. As much as students need to master the NCSOS goals and objectives, they need to master the skills that develop problem solving strategies, as well as the creativity and innovative thinking skills that have become critical in today’s increasingly interconnected workforce and society. The Partnership for 21st Century Skills website is provided below for more information about the skills and resources related to the 21st Century classroom. http://www.21stcenturyskills.org/index.php?option=com_content&task=view&id=27&Ite mid=120 NC SCS Chemistry 1.01 & 3.03 21st Century Skills Communication Skills Conveying thought or opinions effectively Chemistry- Unit 9 DRAFT Activity The Stoichiometry of S’mores 4 When presenting information, distinguishing between relevant and irrelevant information 1.01, 1.03 & Explaining a concept to others 3.03 1.01 & 3.03 Interviewing others or being interviewed Computer Knowledge Using word-processing and database programs Developing visual aides for presentations Using a computer for communication Learning new software programs Employability Skills Assuming responsibility for own learning 1.01 & 3.03 3.03 Persisting until job is completed Working independently Developing career interest/goals Responding to criticism or questions Information-retrieval Skills Searching for information via the computer Searching for print information Searching for information using community members Language Skills - Reading Following written directions Chemistry- Unit 9 DRAFT Stoichiometry Practice Problems Lab: Mass & Mole Relationships in a Chemical Reaction The Stoichiometry of S’mores Stoichiometry Practice Problems Lab: Mass & Mole Relationships in a Chemical Reaction The Stoichiometry of S’mores Lab: Mass & Mole Relationships in a Chemical Reaction All activities Most of the activities can be presented as opportunities for students to follow written directions. 5 The teacher will have to work with most students to develop this skill over time. The following activities are well suited to developing skills in following directions: The Stoichiometry of S’mores Stoichiometry Practice Problems Lab: Mass & Mole Relationships in a Chemical Reaction 3.03 Identifying cause and effect relationships 1.01 – 1.03, & 3.03 Summarizing main points after reading Locating and choosing appropriate reference materials Reading for personal learning Language Skill - Writing Using language accurately Organizing and relating ideas when writing 1.01 – 1.03, 3.03 1.01 – 1.03 & 3.03 Proofing and Editing The Stoichiometry of S’mores Lab: Mass & Mole Relationships in a Chemical Reaction All activities Synthesizing information from several sources Documenting sources Developing an outline Writing to persuade or justify a position Creating memos, letters, other forms of correspondence Teamwork Taking initiative Working on a team Chemistry- Unit 9 The Stoichiometry of S’mores Lab: Mass & Mole Relationships in a Chemical Reaction DRAFT The Stoichiometry of S’mores 6 1.01 – 1.03 & 3.03 Thinking/Problem-Solving Skills Identifying key problems or questions Evaluating results Lab: Mass & Mole Relationships in a Chemical Reaction The Stoichiometry of S’mores Lab: Mass & Mole Relationships in a Chemical Reaction Developing strategies to address problems Developing an action plan or timeline Language (ELP) Objectives for LEP Students: Have students to answer the essential questions in this unit each day in paragraph form. At the end of Unit 9, student will summarize key concepts about stoichiometry. ENGAGE: Provide groups of students with a bag containing plastic knives, spoons and forks. Instruct the students that they are preparing for a dinner party and need to assemble a “set” of dinnerware for each guest. They will assemble a knife/spoon/fork set using the following “recipe” or equation. 2 forks (1 dinner and 1 salad) + 1 knife + 1 spoon = 1 set Instruct the students to answer the following questions: a) How many sets can you assemble from your supply? b) Which is the limiting component? c) What parts are left over after the sets are made? d) How many of each part are left? Lead the students in a discussion regarding chemical equations. Tell them that balanced chemical equations are like recipes for chemists. The reactants are like ingredients such as graham crackers, marshmallows and candy, and the products are like the finished product such as S’mores. Industry uses balanced chemical reactions to predict the amounts (or yields) of many different types of products. To use reactants (or Chemistry- Unit 9 DRAFT 7 reagents) efficiently and cost effectively, the correct amounts of the reactants need to be used to produce the most product for the lowest cost. Teacher Notes: The following is an example set-up for 5 lab groups. Group 1 (2 sets)- 6 forks, 4 knives, 2 spoons (The spoon is the limiting component.) Group 2 (1 set)- 2 forks, 2 knives, 3 spoons (The fork is the limiting component.) Group 3 (1 set)- 3 forks, 3 knives, 3 spoons (The fork is the limiting component.) Group 4 (2 sets)- 5 forks, 1 knife, 3 spoons (The knife is the limiting component.) Group 5 (3 sets)- 8 forks, 4 knives, 3 spoons (The spoon is the limiting component.) This illustrates how in a chemical reaction when one of the reactants is used up, the reaction can no longer continue. EXPLORE: (60 min.) The Stoichometry of S’Mores This activity will allow students to explore the principle of stoichiometry by building S’Mores. They will identify and demonstrate the Law of Conservation of Matter, write and balance a synthesis chemical equation, define and identify the limiting reactant of a reaction, and solve stoichiometry problems relating mass to moles and mass to mass. The activity can be found on the web at the following link: http://devacaf.caes.uga.edu/main/lessonPlan/SMoreLP.pdf This activity was developed through a project called the Science Behind our Food which was supported by a grant through the National Science Foundation. Please see the link below: http://www.uga.edu/discover/sbof/ Note: The extension questions in the activity can be used for honors students to meet objective: 3.03-1 H Evaluate reactions to determine limiting reactant and percent yield. Chemistry- Unit 9 DRAFT 8 Chemistry- Unit 9 DRAFT 9 Chemistry- Unit 9 DRAFT 10 Chemistry- Unit 9 DRAFT 11 Chemistry- Unit 9 DRAFT 12 Chemistry- Unit 9 DRAFT 13 EXPLAIN: Have students to present their answers from the Post-Lab Questions and the Extension Questions to the class. Instruct them to explain their reasoning. Essential Question: How are balanced chemical equations used to compare quantitative amount of reactants and products? ELABORATE: (45 min.) Stoichiometry Power Point The teacher should use the PowerPoint to show students that a balanced equation is necessary when working stoichiometric problems and it is the coefficients that relate the given quantity to the wanted quantity. The PowerPoint includes mole-to-mole conversions, mass-to-mass conversions, and stoichiometry conversions involving gases at STP using the molar volume of a gas, 22.4L/mol. o The conversion factor is in the Chemistry Reference Tables; make sure students know that it is available to them on assignments and assessments. o Guided and independent practice resources are provided in the resource column. The teacher should provide sufficient guided practice for students. Essential Question: How do you calculate the number of moles and the mass of a reactant or product when given the number of moles or the mass of another reactant or product? Slide 1 Stoichiometry Chemistry- Unit 9 DRAFT 14 Slide 2 So what exactly is stoichiometry? Deals with the amount of substances involved in chemical reactions…… reactions…… Both reactants and products Slide 3 I. MoleMole-mole Calculate # moles of one substance that will react or be produced by another substance w/ a given # moles moles A moles B Only ONE conversion factor (mole ratio) *****must always have BALANCED CHEMICAL EQUATION!!!!!!! Slide 4 Sample: CO2 + 2LiOH Li2CO3 + H2O How many moles LiOH are needed to react with 20.0 mols CO2? (40.0 mols LiOH) Where does the mole ratio come from? THE CORRECTLY BALANCED EQUATION!!!!!! Chemistry- Unit 9 DRAFT 15 Slide 5 Practice: 2C2H6 + 7O2 4CO2 + 6H2O 1) If 4.5 moles ethane undergoes combustion, how much oxygen is required in moles? (16 moles) 2) If 7.36 moles CO2 are produced, how many moles of water are produced? (11.0 moles) Slide 6 II. MassMass-mass Calculate the # grams of one substance to react or be produced by given # grams of another substance THREE conversion factors Mass A moles A A moles B mass B Order of conversion factors: 1. inverted molar mass of A 2. mole ratio 3. molar mass of B Slide 7 Practice: Sn + 2HF SnF2 + H2 How many grams of SnF2 are produced from a rxn of 30.0g HF? (118 g SnF2) Chemistry- Unit 9 DRAFT 16 Slide 8 Practice: CaC2 + 2H2O C2H2 +Ca(OH)2 1. How many grams of acetylene are produced by adding water to 5.00g of CaC2? (2.03 g) 2. How many grams of water are needed to produce 347.8g Ca(OH)2? (169.1 g) Slide 9 Beyond 4 Major Types How many molecules of O2 produced from 29.2g water if it is decomposed: 2H2O 2H2 + O2 (4.88 x 1023 molecules of O2) How many grams NO2 must react w/ H2O to produce 5.00 x 1022molecules of NO? 3NO2 + H2O 2HNO3 + NO (11.5 g NO2) Slide 10 Assuming STP, how many liters of oxygen are needed to produce 19.8L of SO3 ? (a gas) 2SO2 + O2 2SO3 (g) (9.90L O2) Chemistry- Unit 9 DRAFT 17 EVALUATE: (60 minutes) Stoichiometry Practice Problems This activity will allow students to EVALUATE their understanding of stoichiometry with guided and independent practice. After students have completed these problems, the teacher will EVALUATE student understanding by going over the problems. STOICHIOMETRY PRACTICE PROBLEMS SHOW YOUR WORK AND BOX YOUR ANSWERS!!! Automotive air bags inflate when sodium azide, NaN3 rapidly decomposes: 2NaN3(s) Na(s) + 3N2(g) 1. How many moles of N2 are produced by the decomposition of 2.50mols of NaN3? 2. How many grams of NaN3 are required to for 6.00g N2(g)? Detonation of nitroglycerin (C3H5N3O9) proceeds as follows: 4C3H5N3O9(l) 12CO2(g) + 6N2(g) + O2(g) + 10H2O(g) 3. How many grams of nitroglycerin are needed to produce 25.0 grams of CO2? 4. How many grams of water are produced by 8.02g of nitroglycerin? The complete combustion of octane, C8H18, a component of gasoline, proceeds as follows: 2C8H18(l) + 25O2(g) 16CO2 + 18H2O(g) 5. How many moles of oxygen are needed to burn 15 moles of octane? 6. How many grams of CO2 are produced by 5.00g of octane? The fermentation of glucose (C6H12O6) produces ethyl alcohol (C2H5OH) and CO2: C6H12O6(aq) 2C2H5OH(aq) + 2CO2(g) 7. How many moles of carbon dioxide are produced when 0.400mol of glucose reacts in this fashion? 8. How many grams of glucose are needed to form 7.50g C2H5OH? Hydrofluoric acid, HF(aq), cannot be stored in glass bottles because compounds called silicates in the glass are attacked by the acid. Sodium silicate (Na 2SiO3), for example acts as follows: Na2SiO3(s) + 2HF(aq) H2SiO3(aq) + 2NaF(aq) 9. How many grams of NaF form when 25.7g of HF reacts with excess sodium silicate? 10. How many grams of Na2SiO3 can react with 0.800gHF? Chemistry- Unit 9 DRAFT 18 11. How many molecules of O2 are contained in 34.66 dm3 of O2? 12. How many moles of O2 are contained in 34.66 dm3 of O2? 13. How many grams of O2 are contained in 34.66 dm3 of O2? 14. How many dm3 of hydrogen would be produced from the decomposition of 124.5 dm3 of water vapor? 15. When reacting sulfur with oxygen, sulfur trioxide gas can be produced. What volume of sulfur trioxide can be produced from 300 cm3 of oxygen and an excess of sulfur? 16. How much hydrogen chloride gas can be produced from an excess of calcium chloride and 33.5 dm3 of water vapor? 17. What volume of water vapor and carbon dioxide are produced by the combustion of 33.6 cm3 of methane? 18. What volume of oxygen would be needed to react with 44.5 dm 3 of nitrogen when producing nitrogen oxide (NO)? ENGAGE: Instruct students to answer the following questions before beginning the lab (Mass and Mole Relationships in a Chemical Reaction). Questions 1. Describe how a geologist determines if a rock contains calcium carbonate. 2. Write the formulas for sodium hydrogen carbonate, hydrochloric acid, carbon dioxide, water, and sodium chloride. 3. Write a balanced equation for the reaction of sodium hydrogen carbonate with hydrochloric acid. 4. How will you know when all of the water has been evaporated from your product? 5. How many moles are present in 585 g of sodium chloride? Show calculation. 6. What is the mass of 3.4 moles of carbon dioxide? Show calculation. EXPLORE: (90 min.) Lab: Mass and Mole Relationships in a Chemical Reaction The students will use this experiment to explore the relationship between the moles and mass values for reactants and products in a chemical reaction. Essential Question: How were you able to determine the mole and mass relationship in this chemical reaction? Chemistry- Unit 9 DRAFT 19 Mass and Mole Relationships in a Chemical Reaction The reaction of a carbonate with an acid is an example of a common chemical reaction. One particular example is the reaction of acetic acid, or vinegar, with sodium hydrogen carbonate, or baking soda. This reaction produces carbon dioxide gas and water. A similar reaction is used by geologists to test rocks for the presence of limestone, or calcium carbonate. A few drops of hydrochloric acid are placed on a sample of rock. If bubbles of gas can be observed, then it is assumed that the rock contains limestone. The reaction of sodium hydrogen carbonate with hydrochloric acid is a convenient reaction to use in studying relationships in chemical reactions. It is convenient because the reaction is fairly rapid and produces three products, one of which is a gas and another of which vaporizes at a fairly low temperature. The final product is sodium chloride, another common chemical, which we know as “salt.” In this experiment, you will observe the reaction between hydrochloric acid and sodium hydrogen carbonate and determine the mass and mole relationships in reactants and products. Pre-lab Read the entire lab before you begin. Make a table to record your data. Answer the pre-lab questions. Questions 7. Describe how a geologist determines if a rock contains calcium carbonate. 8. Write the formulas for sodium hydrogen carbonate, hydrochloric acid, carbon dioxide, water, and sodium chloride. 9. Write a balanced equation for the reaction of sodium hydrogen carbonate with hydrochloric acid. 10. How will you know when all of the water has been evaporated form your product? 11. How many moles are present in 585 g of sodium chloride? Show calculation. 12. What is the mass of 3.4 moles of carbon dioxide? Show calculation. Procedure 1. Put on safety goggles and lab aprons. 2. Obtain a 100-mL beaker. Be sure that it is clean and dry. Measure the mass of the dry beaker. 3. Place about 1.5 to 2.0 grams of sodium hydrogen carbonate in the beaker. Measure the mass of the beaker and its contents. 4. Using a pipet, slowly add 1M HCl to the beaker containing the sodium hydrogen carbonate. It is important that you add the acid slowly so that the reaction does not force some of the reactants out of the beaker. Continue adding the acid until the bubbling stops. Do not add any more acid than is necessary. 5. Place your beaker on a hot plate. Gently heat the beaker until only a dry solid remains. Make sure no water droplets remain on the inside of the beaker. 6. Allow the beaker to cool for five minutes. Measure the mass of the beaker and its contents. Chemistry- Unit 9 DRAFT 20 7. Reheat the beaker and its contents for five minutes. Allow the beaker to cool for five minutes. Measure the mass of the beaker and its contents. 8. Repeat Step 7 until a constant mass is reached. 9. Rinse the residue down the sink. Turn off your hotplate. Clean up your lab station. Calculations Show your calculations in the calculation section of your report. Summarize the answers to your calculations in a Calculations Table. 1. Calculate the mass of sodium hydrogen carbonate that reacted. 2. Calculate the number of moles of sodium hydrogen carbonate that reacted. 3. Calculate the mass of sodium chloride that was produced. 4. Calculate the moles of sodium chloride that was produced. 5. Calculate the experimental mole ratio of NaCl to NaHCO3 by dividing each calculated mole by the smaller of the calculated moles. 6. Using the balanced equation from Pre-lab Question #3, determine the theoretical mole ratio of NaCl to NaHCO3. 7. Determine the percent error for your experimental mole ratio of NaCl produced. % error = [experimental mole of NaCl - theoretical mole of NaCl] x 100 theoretical mole of NaCl 8. Using the mass of sodium hydrogen carbonate that reacted, calculate stoichiometrically the theoretical yield of sodium chloride. 9. Calculate the percent yield for this reaction. EXPLAIN: Have students to present and explain their answers to the calculations section of the lab. ELABORATE: The practice problems below will allow students to ELABORATE on their conceptual understanding by practicing skills related to stoichiometry. Mole Ratio Practice 1) Given this equation: N2 + 3 H2 ---> 2 NH3, write the following molar ratios: a) N2 to H2 b) N2 to NH3 c) H2 to NH3 2) Answer the following questions for this equation: 2 H2 + O2 ---> 2H2O a) What is the H2 to H2O molar ratio? b) Suppose you had 10 moles of H2 on hand and plenty of O2, how many moles of H2O could you make? Chemistry- Unit 9 DRAFT 21 c) What is the O2 to H2O molar ratio? d) Suppose you had 15 moles of O2 and enough H2, how many moles of H2O could you make? 3) Use this equation: N2 + 3 H2 ---> 2 NH3, for the following problems a) If you used 1 mole of N2, how many moles of NH3 could be produced? b) If 5 moles of NH3 were produced, how many moles of N2 would be required? c) If 4.00 moles of H2 were used, how many moles of NH3 would be made? d) If 0.800 moles of NH3 were produced, how many moles of H2 are required? Mass-Mass problems 1. 4 FeCr2O7 + 8 K2CO3 + O2 ---> 2 Fe2O3 + 8 K2CrO4 + 8 CO2 (a) How many grams of FeCr2O7 are required to produce 44.0 g of CO2? (b) How many grams of O2 are required to produce 125.0 g of Fe2O3? (c) If 310.0 g of FeCr2O7 react, how many g of O2 will be consumed? (d) How many g of Fe2O3 will be produced from 310.0 g of FeCr2O7? 2. S + O2 ---> SO2 (a) How many grams of sulfur must be burned to give 110.0 g of SO2 (b) How many grams of oxygen will be required for the reaction in part (a)? 3. 6 NaOH + 2 Al ---> 2 Na3AlO3 + 3 H2 (a) How much aluminum in required to produce 18.7 g of hydrogen? (b) How much Na3AlO3 can be formed from 169.0 g of sodium hydroxide? (c) How many moles of NaOH are required to produce 5 g of hydrogen? 4. BaO + H2SO4 ---> BaSO4 + H2O (a) How much BaSO4 can be formed from 188.0 g of H2SO4? (b) If 62.00 g of water is formed during this reaction, how much BaO was used? 5. NaCl + AgNO3 ---> AgCl + NaNO3 (a) 88.00 g of NaCl should produce how many grams of AgCl? (b) How much AgCl can be produced from 102.0 g of AgNO3? 6. 2 KMnO4 + H2SO4 ---> K2SO4 + Mn2O7 + H2O (a) How many moles of Mn2O7 can be formed from 176.0 g of KMnO4? (b) How many grams of Mn2O7 can be formed from 210.0 g of KMnO4? (c) How much H2SO4 is needed to produce 45.00 g of water? Gas Stoichiometry Practice 1) Calcium carbonate decomposes at high temperatures to form carbon dioxide and calcium oxide: CaCO3(s) CO2(g) + CaO(s) How many grams of calcium carbonate are needed to form 2.45 liters of carbon dioxide? 2) Ethylene burns in oxygen to form carbon dioxide and water vapor: C2H4(g) + 3 O2(g) 2 CO2(g) + 2 H2O(g) How many liters of water can be formed if 3.25 liters of ethylene are consumed in this reaction? Chemistry- Unit 9 DRAFT 22 3) When chlorine is added to acetylene, C2H2Cl4 is formed: 2 Cl2(g) + C2H2(g) C2H2Cl4(l) How many liters of chlorine will be needed to make 85.0 grams of C2H2Cl4? EVALUATE: Sample Assessment Questions: Unit Goal/R Questions BT Tag 9 3.03 C3 1. Determine the mass of water produced from the decomposition of 25.0g of ammonium nitrate, NH4NO3. NH4NO3 N2O + 2 H2O A. 2.8 g B. 5.6 g C. 11.2 g D. 22.4 g C3 2. Determine the volume of the container that holds 2.40 mol of gas at STP. A. B. C. D. Chemistry- Unit 9 13.5 L 27.0 L 54.0 L 108 L DRAFT 23 EVALUATE: (45 minutes) Below are sample test items obtained from the WIZARD test bank developed by eduware™ that can be used to allow students to assess their understanding and abilities and allow the teacher to evaluate the students understanding of key concepts and skill development for this unit. Chemistry- Unit 9 DRAFT 24 Chemistry- Unit 9 DRAFT 25 Chemistry- Unit 9 DRAFT 26 Chemistry- Unit 9 DRAFT 27