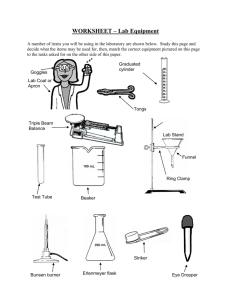
Bunsen Burner Worksheet: Parts, Use, and Heating Efficiency
advertisement

Name________________________ Period ________ Date ____________ Getting to know your Bunsen Burner PURPOSE: - To learn the parts of a Bunsen burner. To learn how to properly use a Bunsen burner. To answer the question “Where is the most efficient height for heating up a liquid quickly using a Bunsen burner?” PROCEDURE: Part 1: Learning the Parts 1. Take out your Bunsen burner and hose. Attach the hose to the gas jet. 2. As I discuss the parts of the Bunsen burner, label them on the diagram below. 3. In your own words, describe the function of each part of the Bunsen burner. Base _____________________________________________________________ Rubber Hose/Gas Inlet _____________________________________________________________ Gas/Needle valve _____________________________________________________________ Air Ports _____________________________________________________________ Barrel _____________________________________________________________ Part 2: Using the burner Make sure the gas jets at your table are turned off before the lab begins. Measure the height of your Bunsen burner from the base to the top. Using a pencil, mark this height on your ring stand. Make a mark 3 cm, 9 cm and 12 cm above the height of your Bunsen burner's height on your ring stand. You should now have 4 marks on your ring stand. 3. Set up a ring clamp and wire gauze square on your ring stand. Adjust the ring so that the bottom of the ring clamp is on the 3 cm mark. 4. Using a graduated cylinder carefully measure out 80.0 ml of water and transfer it into a 250 ml beaker. 5. Place the beaker on the ring stand with the wire gauze under It. Record the temperature of the water in the beaker. This is the temperature at time equals 0 minutes reading. Record it in your data table. 6. Hold the Bunsen burner away from the ring stand setup. Light a match. Hold the lit match 1 inch above the barrel of the Bunsen burner. 7. Have your partner slowly adjust the gas jet until the handle is parallel to the nozzle. 8. With your burner lit, put it under the beaker and use the thermometer to take the temperature of the water every minute for five minutes. Make sure your record the temperatures in your data table. Use the clock for accurate timing. ***Be careful that the thermometer is not resting on the bottom of the beaker when you take the temperature!*** 9. When you are done with the first 5-minute trial, move the burner out of the way, but DO NOT TURN IT OFF! A GROUP MEMBER MUST WATCH THE BURNER AT ALL TIMES. 10. USING BEAKER TONGS, carefully empty the beaker into the sink. Rinse the beaker with cold water from the sink. Remove the wire gauze with your tongs and place it on the table, away from the burner and group members. 11. Use the oven mitt to loosen the thumbscrew so you can adjust the height of the ring. 12. Move the ring to the next height and tighten the thumbscrew. Replace the wire gauze using the beaker tongs. 11. Measure out a new 80.0 ml of water and transfer it to the beaker, place the beaker back on the ring stand, and record the temperature for your 0 minute time. 12. Place the burner under the beaker setup and record the time and temperature every minute for 5 minutes. 13. Repeat the process for the different heights. 1. 2. Data Table for Part 2 Water Temperature at Time Intervals Height From Top of Burner 0 Min 1 min 2 min 3 min 4 min 5 min 3 cm 9 cm 12 cm Data Analysis 1. On one sheet of graph paper, create a line graph containing all 3 data sets. Plot the time on the x-axis and the temperature of the water on the y-axis. Be sure to create a key so we know which line corresponds with which height. Questions 1. Which height was the most efficient at heating the water? ____________ 2. How do you know which height was most efficient at heating the water? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 3. Why do you think the water heated most efficiently at that height? __________________________________________________________________ __________________________________________________________________ _________________________________________________________________ 4. For complete combustion, lots of oxygen is needed. You know you have complete combustion when you have a very hot, blue flame. Describe why the flame looks the way it does with the air ports closed. __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 5. How do you know when the airports are open enough? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 6. Where in the flame should you hold a test tube for most efficient heating? Why? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________