Water Properties Lab: Cohesion, Adhesion, Surface Tension
advertisement
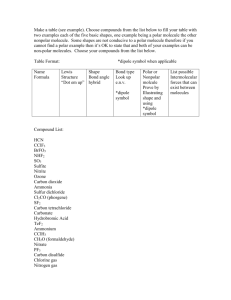
Name ___________________________________ Date _________________________ Per. ______ Observing the Properties of Water Purpose: to observe properties of polar substances (cohesion, surface tension, adhesion, versatile solvent); to observe the differences in properties of polar and nonpolar substances; to identify a substance as polar or nonpolar based on its properties/behavior. Introduction: Water is the most important compound in organisms. The human body is 67% water. 70% of your skin is made of water. You can survive only 5-7 days without water. Water exhibits several properties: a. Polar d. high surface tension g. expands when freezes b. Cohesive e. high heat h. versatile solvent c. Adhesive f. resistance to temperature change In your lab notebooks draw the following diagrams and label the negative and positive areas of each molecule based on where the electrons and protons are located. O H H Water I. Oil Cohesion refers to the ability of a molecule to “stick to itself”. Cohesion can be measured and observed by dropping water (drop by drop) onto a penny. The three liquids we will be using are: water, vegetable oil, and alcohol. 1. Write the scientific question into your notebook: Is ethanol polar or nonpolar? 2. Write an if ….then hypothesis that predicts what you think will happen in this investigation. It will be in this format: “If ____(dependent variable)____ is related to _______(independent variable)___, then _________(predict what ethanol will do on the penny.)________ because ____(explain why in terms of polarity)_________.” 3. Identify the following: Independent variable Dependent variable Control group Controlled variables Methods: 1. Place a clean penny on a paper towel. Slowly drop water using your dropper onto the penny, one drop at a time. Do NOT let the dropper touch the water drops already on the penny. Count the number of drops that fit on top of the penny. Record this in a data table. Don’t forget to title your table using a complete descriptive sentence. Make sure you have descriptive headings above each column. Repeat this procedure 2 more times and take the average. Record each trial and the average in your data table. For qualitative results: Draw a side view sketch of what the penny looked like with the water on it and describe what you saw. 2. Now try the same experiment using oil. 3. Repeat the same procedure using the ethanol. II. Surface tension – 1. Write an if ….then hypothesis that predicts what you think will happen in this investigation. It will be in this format: “If ____(dependent variable)____ is related to _______(independent variable)___, then _________(predict if the paperclip will lay on top of the ethanol.)________ because ____(explain why in terms of polarity)_________.” 2. Identify the following: Independent variable Dependent variable Control group Controlled variables Methods: 1. Take a small paperclip and gently lay it horizontally on the surface of the plain water in a petri dish. Keep trying! Look carefully at the surface of the water just where the paperclip is floating. Record your observations and a sketch in a new data table. 2. Repeat the surface tension experiment using the vegetable oil and then the ethanol instead. Record all of your observations in your data table. III. Capillary Action – Adhesion is the ability of water to stick to polar surfaces. Adhesion helps carry water from roots to leaves (against gravity). Water pulls itself up the sides of the glass by its adhesion to the surfaces of the glass and by the cohesion among water molecules. 1. Write an if ….then hypothesis that predicts what you think will happen in this investigation. It will be in this format: “If ____(dependent variable)____ is related to _______(independent variable)___, then _________(predict what ethanol will do in the capillary tube.)________ because ____(explain why in terms of polarity)_________.” 2. Identify the following: Independent variable Dependent variable Control group Controlled variables Methods: 1. Obtain a clean capillary tube from your teacher. 2. Place 3 drops of water on one end of a glass microscope slide, 3 drops of oil in the middle, and 3 drops of ethanol on the other end, use the properly labeled pipet for each!! They cannot touch. If you can’t fit them all on one slide, then use a second slide. 4. Put one end of the capillary tube in the water. Watch what happens after ten seconds. Repeat with the other 2 liquids, but use a new capillary tube each time. 6. Record your observations in a new data table. 7. When you finish, wash all of your slides with soap and lay them to dry as indicated by your teacher. Hand her your capillary tubes also. IV. Versatile Solvent – “Like dissolves like”, meaning that polar substances will dissolve other polar (and ionic) substances, and nonpolar substances will dissolve other nonpolar substances. If one liquid is polar and the other is nonpolar, they will not mix – think of mixing oil and water. 1. Write an if ….then hypothesis that predicts what you think will happen in this investigation. It will be in this format: “If ____(dependent variable)____ is related to _______(independent variable)___, then _________(predict if ethanol will dissolve in water or oil)________ because ____(explain why in terms of polarity)_________.” 2. Identify the following: Independent variable Dependent variable Control group Controlled variables Methods: 1. Add 2mL of each solution in the following way to 3 small test tubes: Tube #1 – oil and water Tube #2 – ethanol and water Tube #3 – ethanol and oil 2. Gently swirl each. Write your observations and a labeled sketch into a new data table.