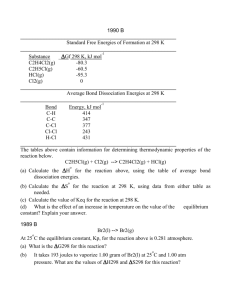
PM S3 K7 Define the Molar Heat of Combustion and calculate the

PM S3 K7 Define the Molar Heat of Combustion and calculate the value for ethanol from first hand data.
Formula: density = mass / volume kJ mol -1 / molar mass = kJ g -1 kJ g -1 x molar mass = kJ mol -1
Q1 Define the molar heat of combustion of a compound:
The amount of heat energy released when one mole of a compound is completely combusted under standard conditions.
Q2 The following table contains information about different fuels. Complete the table.
Fuel Formula Heat of combustion
(kJ/mol)
Heat of combustion
(kJ/g)
Hydrogen H
2
285 141
Coke (carbon)
Methane
Ethane
Propane
C
CH
4
C
C
2
3
H
H
6
8
394
890
1560
2220
32.8
55.5
51.9
50.3
Methanol
Ethanol
CH
3
OH
C
2
H
5
OH
727
1370
22.7
29.7
Q4 Identify the fuel which would produce the most heat by the combustion of 1g of the fuel.
Hydrogen
Q4 Which homologous series do methane, ethane and propane belong to?
Alkanes
Q5 The molar heats of combustion of methane, ethane and propane are very different but the heat of combustion per gram of each of these fuels is very similar. Why is this?
One mole of propane contains more matter (atoms) than one mole of methane as propane molecules are larger than methane molecules. Therefore there will be more CO
2 and H
2
O and energy released when one mole of propane is combusted.
They have similar energy contents per gram because they are essentially the same substance: hydrocarbons, containing C n
H
2n+2
atoms and they will have similar densities.
Therefore 1g of each will contain similar numbers of C and H atoms and therefore will release similar amounts of CO
2,
H
2
O and energy when combusted.
Q6 The density of methanol is 0.790 g/mL. What does this mean? (look at the units)
Density is the amount of mass per volume
Q7 Using the formula for density (which you need to remember), calculate the mass of 1.000L of methanol. d= m/v ; m = d x v = 0.790 g/mL x 1000mL = 790g
Q8 A model aeroplane runs on methanol and has a fuel tank which holds 230mL. What mass of methanol would be contained in a full tank? d= m/v ; mass = d x v = 0.790 g/mL x 230g = 181.7g = 182g (3s.f.)
Q9 Using the table of data, calculate the amount of energy contained within 230mL methanol.
Heat of combustion for methanol = 22.7 kJ/g; every gram contains 22.7kJ.
So, using the mass from before (unrounded) 181.7g x 22.7kJ/g = 4124.59 kJ = 4120kJ (3sf)
Q10 A rally car holds 90.0L of ethanol. The density of ethanol is 0.780 g/mL.
The rally car can travel 100km for every 10.0L of fuel.
How much energy does it use per km?
Mass of 90.0L of ethanol = density x volume = 0.780 g/mL x 90000mL = 70200 g
Energy contained = 70200 g x 29.7 kJ/g = 2084940 kJ.
The car will do 900km on a full tank.
So the amount of energy per km = 2084940 kJ / 900km = 2316.6 kJ / km = 2320 kJ/km (3sf)