4 - Chemical Paradigms
advertisement

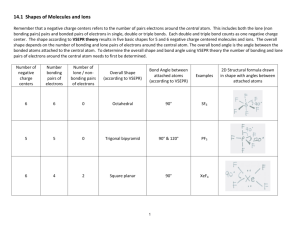
4.2.7 & 4.2.8 Shapes, and bond angles for molecules with two, three and four negative charge centers The shape of a molecule has an important part to play in determining its chemical (e.g. reactivity and pH) and physical properties (e.g. mpt and bpt). A negative charge center or region refers to the number of pairs electrons around the central bonded atom. This includes both the lone (non bonding pairs) pairs and bonded pairs of electrons in single, double or triple bonds. Each double and triple bond counts as one negative charge center. Watch out for non octet examples. Number of negative charge centers Shape with respect to the number of negative charged centers Bond Angle with respect to negative charge centers 2 Linear 180° 3 Trigonal planar 120° 4 Tetrahedral 109.5° Valence shell electron pair repulsion theory (VSEPR) GN Lewis proposed that chemical bonds resulted when two atoms shared a pair of electrons. The Lewis concept allowed for "electron dot bookkeeping" in the form of a Lewis dot digram to show how atoms could share electrons to achieve their quota as a noble gas or an octet of electrons. Lewis originally did not set out to describe the shape of the molecule but it soon became apparent to him that the electron pairs around the central atom, being like charged, would repel each other. This lead to his VSEPR theory which is still used today to describe the shape of a molecule. In VSEPR theory lone pairs of electrons are closer to the central atom and therefore closer to one another than the bonding pairs of electrons because they are being attracted by the positive protons in the nucleus of one atom instead of two. The lone pairs and bonding pairs of electrons are arranged around the central atom so as to minimize the repulsion between the lone and bonding pairs of electrons. The relative magnitude of the electron pair repulsions is: Lone pair / lone pair > bonded pair / lone pair > bonded pair / bonded pair repulsion The overall shape according to the VSEPR theory depends on the number of bonding pairs and lone pairs of electrons around the central atom of a molecule. The overall bond angle is the angle between the bonded atoms attached to the central atom. 1 To determine the overall shape and bond angle of a molecule by the VSEPR theory the number of bonding and lone pairs of electrons around the central atom need to be identified. Number of negative charge centers Number of bonding pairs of electrons Number of lone pairs of electrons Overall Shape (according to VSEPR) Overall Bond Angle (according to VSEPR) 2 2 0 Linear 180° 3 3 0 Trigonal planar 120° 4 4 0 Tetrahedral 109.5° 4 3 1 Trigonal pyramid 107º 4 2 2 Bent / V shape 104.5º Reason for the shape (according to VSEPR) To minimize the repulsion between them, the two bonding pairs repel each other equally To minimize the repulsion between them, the three bonding pairs repel each other equally To minimize the repulsion between them, the four bonding pairs repel each other equally To minimize the repulsion bonded pair-lone pair > bonded pairbonded pair repulsion To minimize the repulsion Lone pair-lone pair > bonded pairlone pair > bonded pair- bonded pair repulsion Examples CO2, HCN, BeCl2 BF3, C2H4 CH4, SiCl4, CCl4 NH3, NF3 H2O, H2S NOTE: The number of negative charge centers may not be reflected in the molecular shape. If the electrons are not being used for bonding then they cannot be seen and the molecule is described as if they were not there. In other words the shape and angle of the molecule is determined with respect to the attached atoms. 2 Complete the column. Formula and name HCN Hydrogen cyanide BeCl2 Beryllium chloride table below leaving out the last Structural formula in correct shape (To do this you may need to the Lewis dot diagram first) H-C≡N Cl-Be-Cl Overall Number Bond negative Angle charge (according centers to VSEPR) 2 2 180 180 Overall Shape (according to VSEPR theory) Explanation for the overall shape using VSEPR theory linear 2 bonding pairs of electrons & 0 lone pairs around the central atom. In order to minimize the repulsion the bonding pairs of electrons repel each other equally. linear 2 bonding pairs of electrons & 0 lone pairs around the central atom. In order to minimize the repulsion the bonding pairs of electrons repel each other equally. 3 Polar or non polar covalent Formula and name CO2 H2O CHCl3 NH3 Structural formula O=C=O Number negative charge centers 2 4 4 4 Bond Angle 180 104.5 109.5 107 Overall Shape Explanation for shape using VSEPR theory linear 2 bonding pairs of electrons & 0 lone pairs of electrons around the central atom. Bonding pairs of electrons repel each other equally in order to minimize the repulsion between them. OR 2 negative charge centers repel each other as much as possible to minimize the repulsion between them. bent 2 bonding pairs of electrons and 2 lone pairs of electrons. In order to minimize the repulsion between then the 2 lone pairs of electrons exert a greater repulsion than the bonding pair and lone-bonding pair repulsion. 4 bonding pairs of electrons and 0 lone pairs of electrons around the central atom. To minimize the tetrahedral repulsion between them, the four bonding pairs repel each other equally 3 bonding pairs of electrons and 1 lone pair of electrons around the central atom. To minimize the repulsion between them the bonded pair / lone pair > bonded pair / bonded pair repulsion trigonal pyramid 4 Polar or non polar Formula and name Structural formula Number negative charge centers Bond Angle Overall Shape Explanation for shape using VSEPR theory H2S CH4 BF3 SiCl4 5 Polar or non polar Formula and name Structural formula Number negative charge centers Bond Angle Overall Shape Explanation for shape using VSEPR theory CClF3 Cl2O C2H2 ethyne H2CO methanal 6 Polar or non polar Formula and name Structural formula Number negative charge centers Bond Angle Overall Shape Explanation for shape using VSEPR theory SO2 Sulfur dioxide C2H4 ethene POCl3 O3 7 Polar or non polar CH3NH2 Aminomethane (C and N) (C and N) ( with respect to C and N) Extra Problems 1. Predict the bond angle and shape around the carbon atom and the oxygen atom attached to the hydrogen atom in methanoic acid (HCOOH). 2. Compare the bond length and strength between the carbon and oxygen atoms in carbon dioxide and carbon monoxide. 3. State and compare the difference in the bond angle in CH4 and NF3. 8 4. Compare the N-N-H bond angle between the two possible Lewis structures of N3H. 5. State the bond angle around the carbon and nitrogen atoms in aminomethane, CH 3NH2 6. Draw the structural formula for ethanoic acid and determine its relative molecular mass. 9 ANSWERS Formula HCN Hydrogen cyanide BeCl2 Beryllium chloride Structural formula H-C≡N Cl-Be-Cl Number negative charge centers 2 2 Shape with respect to negative charge centers linear linear Bond Angle 180º 180º Overall Shape Explanation for shape using VSEPR theory Polar or non polar linear 2 bonding pairs of electrons & 0 lone pairs around the central atoms. In order to minimize repulsion the bonding pairs of electrons repel each other equally. polar linear 2 bonding pairs of electrons & 0 lone pairs around the central atoms. Bonding pairs of electrons repel each other equally. Non polar CO2 O=C=O 2 linear 180º linear C2H2 ethyne H-C≡C-H 2 linear 180º linear 3 Trigonal planar 120º Trigonal planar BF3 10 2 bonding pairs of electrons & 0 lone pairs of electrons around the central atom. Bonding pairs of electrons repel each other equally. OR 2 negative charge centers arranged as far apart as possible. 2 bonding pairs of electrons & 0 lone pairs of electrons around the central atom. Bonding pairs of electrons repel each other equally. OR 2 negative charge centers arranged as far apart as possible. 3 bonding pairs of electrons & 0 lone pairs of electrons around the central atom. Bonding pairs of electrons repel each other equally. OR 3 negative charge centers arranged as far apart as possible. Non polar Non polar H2CO methanal 3 SO2 Sulfur dioxide 3 C2H4 ethene 3 for each C atom CH4 methane 4 SiCl4 4 ClF3 H2O 4 4 3 bonding pairs of electrons & 0 lone pairs of electrons around the central Trigonal Trigonal atom. Bonding pairs of electrons repel 120º planar planar each other equally. OR 3 negative charge centers arranged as far apart as possible. 2 bonding pairs & 1 lone pair of electrons Trigonal around the central atom. Lone pair bent ~117º planar bonding pair repulsion is greater than bonding pair – bonding pair repulsion. 3 bonding pairs & 0 lone pairs of electrons around each C atom. 3 Trigonal 120º bonding pairs of electrons repel each planar for between Trigonal other equally in order to minimize the each C each H planar repulsion. OR atom atom 3 negative charge centers on each carbon atom arranged as far apart as possible to minimize repulsion. 4 bonding pairs & 0 lone pairs of electrons. In order to minimize the Tetrahedral 109.5º Tetrahedral repulsions the 4 bonding pairs of electrons repel each other equally. Tetrahedral Tetrahedral Tetrahedral Polar Polar Non Polar Non Polar 109.5º 4 bonding pairs & 0 lone pairs of Tetrahedral electrons. 4 bonding pairs of electrons repel each other equally. Non Polar 109.5º 4 bonding pairs & 0 lone pairs of Tetrahedral electrons. 4 bonding pairs of electrons repel each other equally. Polar 104.5º 2 bonding pairs of electrons and 2 lone pairs of electrons. The 2 lone pairs of electrons exert a greater repulsion than the bonding pair and lone-bonding pair repulsion. Polar Bent 11 NH3 ammonia 4 POCl3 3 or 4 Tetrahedral Trigonal pyramid 107º Tetrahedral 100-112 Tetrahedral (C and N) CH3NH2 Aminomethane 4 3 bonding pairs and 1 lone pair of electrons. Lone pair – bonding pair repulsion is greater than bonding pair repulsion. Tetrahedral C (109.5º) N (107º) Polar Carbon - 4 bonding pairs & 0 lone pairs of electrons. 4 bonding pairs of electrons repel each other equally. C Tetrahedral N N - 3 bonding pairs and 1 lone pair of Trigonal electrons. Lone pair – bonding pair pyramid repulsion is greater than bonding pair repulsion. 12 Polar Polar IB Problems 1. Predict the bond angle around the carbon atom and the oxygen atom attached to the hydrogen atom in methanoic acid (HCOOH). Carbon – 3 bonding pairs and 0 lone pairs of electrons. Bond angle 120º (trigonal planar shape) Oxygen – 2 bonding pairs of electrons and 2 lone pairs. Bond angle 104.5º (bent shape) 2. Compare the bond length and strength between the carbon and oxygen atoms in carbon dioxide and carbon monoxide. The more bonding electrons between the nuclei of the two bonded atoms, The greater the attraction the protons in the nucleus of each atoms have for the bonding electrons the shorter the bond and the more energy will be required to break it. Therefore the C=O bond in CO2 is longer and has a lower bond energy than the C≡O bond in CO. 3. State and compare the difference in the bond angle in CH4 and NF3. CH4 – the bond angle between the H atoms is 109.5º due to 4 bonding pairs & 0 lone pairs of electrons. 4 bonding pairs of electrons repel each other equally in order to minimize the repulsion between them.. NF3 - 107º due to 3 bonding pairs and 1 lone pair of electrons. Lone pair – bonding pair repulsion is greater than bonding pair repulsion, which results in the bonding pairs of electrons being pushed closer together than in CH 4, decreasing the bond angle between the attached atoms. 4. Compare the N-N-H bond angle between the two possible Lewis structures of N3H. 13 5. State the bond angle around the carbon and nitrogen atoms in aminomethane, CH 3NH2 C - 109.5º N - 107º 6. Draw the structural formula for ethanoic acid and determine its relative molecular mass. 14