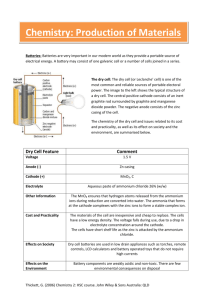
Science Tracks 7 Teacher Resource CD-ROM
advertisement

SCIENCE TRACKS 7 TEACHER RESOURCE CD-ROM © G Thickett, J Stamell, L Thickett 1999 Published by Macmillan Education Australia SAMPLE CHAPTER The Science Tracks Teacher Resource CD-ROMs contain Microsoft Word documents to print out (or view on screen), organised by chapter in the student book, each divided into four parts: Part 1 Answers to text questions in the Science Tracks textbooks. Part 2 Additional topic tests for use in class assessments. Answers are supplied. Part 3 Additional activities and exercises, especially on language and literacy. With answers. Part 4 Selected text and diagrams—print masters to be photocopied or pasted into applications for computing exercises. This file contains a sample chapter from the CD-ROM, Chapter 1 Investigations. It includes diagrams (graphics). To view on screen, make sure that the Show Drawings box in the View tab of Options, and the Drawing Objects box in the Print tab, are ticked. A full contents list of the rest of the chapters on the CD-ROM is given at the end. The chapter contains Bookmarks to enable you to jump quickly to desired sections. Use Word Help for instructions on using Bookmarks. This material is the copyright of the authors, and protected by law. It is provided for the purpose of review and evaluation only. You may not make copies of it. Sales enquiries Enquiries about price and availability should be directed to our Customer Service department by phone on 1 300 135 113 or by fax on Freefax 1 300 135 103 or by e-mail to customer.service@macmillan.com.au. Other enquiries in NSW: Call our NSW State Manager, phone 02 9264 0522, fax 02 9264 0770, or e-mail to rex.parry@macmillan.com.au. Queries to the authors about use of the material in the classroom You may e-mail queries to the authors c/o Rex Parry, Publishing Director, (rex.parry@macmillan.com.au, or use the fax number below). If you want the authors to call you, please indicate a convenient time. Publication details of the Science Tracks 7 Teacher Resource CD-ROM CD-ROM for IBM Windows or Macintosh, files in MS Word 6.0. Price: $72.95 approx, ISBN: 07329 5595 5 Teacher Resource CD-ROMs to accompany Science Tracks 8-10 will be published in late 2000 and early 2001. The licence for the Teacher CD-ROM is an institutional licence. The files may be printed out and photocopied, or copied and pasted into other documents according to Licence Conditions of Use stated in the disk. The files may be copied onto a number of computers, within the purchasing school (one campus only). The CD-ROM is a Shared Network Resource, ie the files may be copied onto a server from where they can be accessed over a network (within that campus only) by computers with MS Word installed. Science Tracks 7 Teacher Resource CD-ROM CHAPTER 1: INVESTIGATIONS CONTENTS Part 1 Answers to text questions Knowledge and understanding (pages 8–9) Language focus (pages 9–10) Problem solving 1.1: Safety rules (pages 10–11) Activity 1.1: Making and recording observations (pages 12–13) Practical activity 1.4: Using a Bunsen burner (pages 13–15) Practical activity 1.5: The hottest part of the flame (pages 15 –17) Practical activity 1.6: Boiling water (pages 18–19) Homework set 1.1: Observing and safety (pages 19–21) Knowledge and understanding (pages 26–27) Language focus (page 27) Problem solving 1.2: Laboratory techniques (pages 28–29) Problem solving 1.3: Reading scales (pages 30–31) Problem solving 1.4: Using tables and graphs (pages 34–37) Homework set 1.2: Using your skills (pages 37–39) Spelling (page 39) Topic test (pages 40–41) 1.3 1.3 1.4 1.4 1.4 1.6 1.6 1.7 1.7 1.8 1.9 1.9 1.11 1.12 1.13 1.13 Part 2 Additional topic test Additional topic text: Recall of knowledge Answers to additional topic text: Recall of knowledge 1.16 1.21 Part 3 Additional activities and exercises A1.1 Activity: How well can you observe? A1.2 Exercise: Additional problem solving questions A1.3 Literacy exercise: Three level reading exercise—the Bunsen burner A1.4 Literacy exercise: Sources of scientific words A1.5 Puzzle: Spelling/wonderword—investigations A1.6 Literacy and numeracy exercise: Puzzleword A1.7 Literacy exercise: Crossword puzzle A1.8 Literacy exercise: Barrier exercise (oral activity) Answers to additional activities and exercises 1.24 1.26 1.29 1.31 1.33 1.34 1.35 1.36 1.39 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Part 4 Selected text and diagrams—print masters Figure 1.15 Match the haz-chem symbols to their descriptions and meanings (Problem solving 1.1) The Bunsen burner Figure 1.55 Complete this column graph (Problem solving 1.4) Figure 1.56 Complete this sector graph (Problem solving 1.4) Figure 1.57 Complete this bar graph (Problem solving 1.4) 1.43 1.44 1.45 1.46 1.47 A full contents list of the rest of the chapters on the CD-ROM is given at the end of this file. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.3 PART 1 ANSWERS TO TEXT QUESTIONS Page numbers refer to pages in the textbook. Knowledge and understanding pages 8–9 1 Most accidents can be avoided by using common sense. 2 If you have an accident, tell the teacher immediately. 3 a Long, loose hair can catch fire easily. b Leather shoes protect feet from spills of corrosive liquids. c Safety goggles protect the eyes from damage. d The contents cannot injure anyone if they explode out of the test tube. e Running in the laboratory can cause an accident. 4 Mistake Correction a Uses her hand to transfer chemicals Uses a spatula b Looks down into the test tube Points the test tube away from all students c Leaves desk and talks to friends Remains at desk while completing the experiment d Places nose directly over the bottle to sniff Use hand to waft air containing the smell of the chemical towards her nose e Glassware close to the edge of the table Set up glassware away from the edge f Thumb over the top of the test tube and the contents shaken up and down Gently tap the test tube on the hand to mix the contents g After boiling picks up beaker with hand Allows the beaker to cool before picking it up and pouring the contents down the sink h solid wastes into the sink Water down the sink and solid wastes in the bin provided by the teacher Language focus pages 9–10 1 a b c d 2 a b c d e analyse: examine minutely, find or show structure corrosive: tending to destroy, wear away or destroy gradually toxic: poisonous contaminate: pollute, infect When working around naked flames, long hair should be tied back. At the end of an experiment, clean up your bench. Before the bell rings, put away all your equipment. During hazardous experiments, goggles or safety glasses should be worn. Throwing things is not allowed in the room. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.4 Problem solving 1.1: Safety rules pages 10–11 PM 1 Haz-chem labels—correct matches: AKP BMU CHQ DIR EJT FNS GLO 2 a Rules will vary with each class. b Class discussion to come to some agreement. c Poster making—attach haz-chem labels to poster. 3 a By pouring this way, no liquid will be able to flow over the label and obliterate the name; any spills which run down the bottle will always be on the side away from the label and therefore not contaminate the skin. b When pouring from the bottle to the cylinder the technician is watching that she adds the correct quantity of liquid to the cylinder. She avoids parallax error. 4 Class discussion of safety issues—examples: Talking on the mobile phone instead of observing carefully. Too many experiments being done at once. Heating near a curtain so it catches fire. Fighting in the lab can cause accidents. Do not eat in the lab. Do not boil a liquid in a vessel with a stopper—an explosion can occur. Activity 1.1: Making and recording observations pages 12–13 1 a b c 2 a b c Taste—it is dangerous to taste chemicals. She needed to make her observations quickly as the reaction occurred. present tense Too difficult to make corrections. A pencil should always be used for drawings. For clarity. Too small a diagram makes it difficult to see enough detail. Accurate drawing required. Practical activity 1.4: Using a Bunsen burner pages 13–15 Prelab 1 2 3 4 Fibre mat protects the desk from heat. closed The barrel—it is near the flame. Turn off the gas immediately and try to relight the Bunsen. If this fails, report the problem to the teacher. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.5 Results and questions 1 a b c d e f g h i j k l m 2 a b 3 a laboratory heat metal rubber gas air open burning smoke plenty closed sooty yellow The yellow flame, because it is easily seen when not in use. Because it is easily seen when not in use. b The blue flame should be used for heating. 4 Heat-proof mat protects the surface of the desk from heat. 5 After turning on the gas, you have to wait a few seconds so that the gas can push out the air which is in the hose. This can blow out your match if you try to light the Bunsen too soon. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.6 6 7 The strength of the flame can be adjusted by: changing the size of the air hole in the collar of the Bunsen to alter the amount of air entering, or changing the flow of gas by using the gas tap. Practical activity 1.5: The hottest part of the flame pages 15–17 Prelab 1 2 3 4 One-third full or 5 cm. The Bunsen hole should be closed. The wooden test-tube holder might burn if it is too close to the flame. If you leave the contents of the tube still, heat will build up in one section and the contents are more likely to explode out. Results and questions 1 Students fill in the table. 2 Must keep everything the same, except for the position of the heating. Keep as many variables constant as possible. 3 Water could explode out of the test tube, it should be pointed away from everyone. 4 It gets too hot; the contents could boil out onto your hand. 5 The test tube heated with the yellow flame became covered with black soot. 6 You should always use safety glasses when doing experiments. Practical activity 1.6: Boiling water pages 18–19 Prelab 1 The blue flame—it is the hottest. 2 Goggles, long hair tied back, heat mat, place equipment away from desk edge, etc. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.7 Homework set 1.1: Observing and safety pages 19–21 1 a, b, c various answers d sight, hearing 2 a various answers b sight, smell, touch c Student to provide drawings. 3 Examples include: astronaut is a male; astronaut is weightless or floating; astronaut has a watch on his left wrist; the cabin is narrow; there is no floor; there are instruments on most surfaces. 4 a Aim: To observe powdered sulfur as it burns in air. b Method: 1. Collect equipment needed. 2. Place 1 cm of water in the gas jar. 3. Collect the sulfur and place it on the deflagrating spoon. 4. Heat sulfur in a blue Bunsen flame until it just begins to burn. 5. Immediately place the deflagrating spoon and burning sulfur into the gas jar. 6. Leave the spoon and its lid in place until the sulfur stops burning. 7. Record all observations. c Student observations: Sulfur started to burn with a purple flame. Whitish, choking fumes produced. The gas jar glows. When the flames go out, the smoke begins to disappear. When removed from the gas jar, the sulfur begins to burn again. d tripod, gauze e Joe should have left the Bunsen on the safety flame so that his group could see it easily. f Use a spatula to place some sulfur on a piece of folded paper. Pour the sulfur into the deflagrating spoon from the paper. g Catherine took the lid off the bottle and carried the open bottle back to her desk. h Other things: not wiping up spills not following the directions of the teacher not recording results while the experiment is proceeding talking to the other groups no safety glasses i The glasses are needed to protect the eyes from burning sulfur. j Joe does not follow instructions. Joe should learn to follow the instructions exactly as told, not to make up his own experiment and not to talk to students at other desks. k Claude does not do any of the experiment. Claude should try to participate more in practical lessons as he will learn valuable new skills. Knowledge and understanding pages 26–27 1 A clean spatula is needed so that the chemicals in the jar do not become contaminated. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.8 2 3 Hold with little finger, or place inverted on a desk. 4 The stirring rod directs the flow of liquid from beaker to beaker. The liquid runs down the rod. 5 Liquid will be allowed to get on the desk. 6 Place it back into its bottle or stand it upright in an empty beaker. 7 If the liquid comes above the filter paper, unfiltered liquid will flow between the paper and glass; some residue will then flow into the beaker below. 8 Yes. Unfiltered liquid will flow though the hole in the paper. 9 The solid could stick to the wet sides of the test tube and not get into the liquid at the base of the test tube. 10 a There may be poisonous liquid or solid on the opening. Toxic gas may be given off. b Tap the test tube with your fingers. c Use the stopper when no gases are being formed or to mix substances thoroughly by shaking. Language focus page 27 1 A doctor uses a spatula to press the tongue down or to one side. 2 reagent: a substance used to cause a reaction contaminate: pollute dilute: weaken by addition of water or other solvent parallax: apparent displacement of an object caused by actual change of point of observation 3 a qualitative: looking at the features of the object or reaction quantitative: measuring the amount of substance reacting 4 a qualitative b quantitative © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.9 5 Meniscus: curved upper surface of a liquid in a container (memisko means a crescent, as of the moon). The thin crescent moon looks like the top of the water. Problem solving 1.2: Laboratory techniques pages 28–29 1 © Any spills which may run down the sides of the bottle will always be away from the label, and therefore if you pick up the bottle with the label against your hand you will not get the spills on you. 2 X: folded in quarters Y: fluted paper The fluted paper allows the liquid to flow more quickly. 3 mass of container + salt 93.2 g mass of container 85.9 g 7.3 g mass of salt is 7.3 g 4 a measuring cylinder 3.4 mL beaker 35 mL b The beaker is less accurate because it does not have accurate scale markings on it. 5 a reading at A = 3.5 cm reading at B = 3.7 cm b The readings are different because of the parallax error. c The eye should be placed in line with the edge of the object to be measured—i.e. at B. d position C Problem solving 1.3: Reading scales pages 30–31 1 a b c 2 a b c d e f 19.4oC 21.3oC Increase in temperature is 1.9oC 5.5 42 65 30 96 50 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.10 3 16 volts 4 5 a 11 kg b 6 The measuring cylinder—it has the most accurate scale markings. 7 Thermometer reading at 4 pm is 1oC. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.11 Problem solving 1.4: Using tables and graphs pages 34–37 PM 1 a b c d e 2 a b 3 a b c d e 4 a b c d e every two minutes made from the water of the same kettle or the same boiling; to control all the factors covered 65oC; uncovered 62oC at 8.5–9 minutes after the start of the experiment 100 hectares = 250 acres 300 acres = 120 hectares Average annual temperature at 70o N is –11o C 26oC 12oC The south pole is colder. 23o – 6o C = 17oC difference Categories along the horizontal axis from left to right: Motor vehicle crash; Medical; Suicide; Other causes; Drowning; Accidental falls. (Note that Figure 1.55 in the textbook is provided as a photocopy master on this disk.) Cause of accident Number of deaths Causes of death of 15–24 year olds Categories from the largest sector clockwise to smallest: Motor vehicle crash; Medical; Suicide; Other causes; Drowning; Accidental falls. (Note that Figure 1.56 in the textbook is provided as a photocopy master on this disk.) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.12 5 a b ‘Hit in the rear’ is the most common accident. c To allow yourself time to break and stop the forward momentum of the car without hitting the car in front. 6 a 1 b slowly c double d four e twenty-five times Homework set 1.2: Using your skills pages 37–39 1 a b c d balance, measuring cylinder, beaker, spatula 3g 150 mL 1. Weigh a clean beaker. 2. Weigh 3 g of salt. 3. Measure out 150 mL of water. 4. Pour the water into the beaker containing the salt and mix. 2 Many answers possible 3 a quantitative b qualitative c quantitative d quantitative e qualitative 4 a 1.1 g b 3.5 g c cow’s milk—has 720 mg of total mineral d 3 times e 35 g f 40 g g lactose, vitamin C © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.13 5 a The lower the water temperature, the longer the time required for the salt to dissolve. b The graph confirms the answer in a. Spelling page 39 1 technique science apparatus reagent hazard prediction 2 nature Topic test pages 40–41 1 B 2 C © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.14 3 Mollie’s mistakes What she should have done a Overfilled the beaker Beaker only one-half to one-third full b Used paper to light Bunsen Use only taper, match or lighter provided c Used smoky flame: Bunsen air hole closed Use blue flame: air hole open d Left experiment to talk to friends Always stay with your experiment/group e Pulled tap hose and knocked over the equipment Be very careful with hot objects, even in the clean-up f Equipment too close to the edge of desk Set up equipment away from desk edges g Friends ran around after accident Stay at desk or help injured friend—cold water on burns h Cried Tell the teacher immediately an accident happens 4 a b c d e 5 hearing touch sight smell taste 6 a The pelican has a long beak, is pale-coloured and has a long neck. The hawk has a short beak, is darker and mottled, and has a short neck. b The pelican’s webbed feet are for swimming. The hawk’s clawed feet are for perching and catching prey. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Answers 1.15 7 a Martin should use a stirring rod, and pour the liquid down the rod from one beaker to the other. b Weigh the watch glass; zero the reading; remove the watch glass; use a spatula to spoon blue crystals onto the watch glass; reweigh the glass. c Make sure eyes are level with the surface of the liquid. 8 a 4g b 2 g removed 9 a 22.5 metres/second b 38 Newtons c 19.7oC © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.16 PART 2 ADDITIONAL TOPIC TEST: RECALL OF KNOWLEDGE Questions 1–5 are multiple choice. 1 Which group of laboratory equipment contains only items made of glass? A gauze, beaker, measuring cylinder B beaker, test tube, tripod C beaker, test tube, measuring cylinder D clamp, retort stand, tripod 2 Which of the following is the first step you must complete when you light a Bunsen Burner? A Light the match. B Turn on the gas tap. C Attach the hose of the Bunsen Burner to the gas tap. D Put the lighted match at the top of the Bunsen Burner. 3 Which sense is not used very often in the laboratory? A smell B taste C touch D sight 4 What is the reading on the following scale? A 12.5 B 12.75 C 13.5 D 13.0 5 Peter was asked to prepare 250 mL of sugar water which has a strength (concentration) of 5 g/100 mL. What pieces of laboratory equipment will he need to complete this task? A measuring cylinder, electric balance B measuring cylinder, electric balance, beaker C measuring cylinder, electric balance, beaker, spatula D measuring cylinder, spatula, beaker © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.17 6 Match the following haz-chem labels with their meanings. (Draw a line from each label to its meaning.) 7 Below is a diagram of a blue Bunsen flame. Draw an arrow to indicate the area of the hottest part of the flame. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.18 8 Jon draws a diagram of equipment he used to evaporate some blue liquid. He has made many mistakes, including spelling. Redraw the diagram correctly and correct any spelling mistakes. 9 Match the following words with their meanings by drawing a line from the word in column A to its meaning in Column B. Column A Column B Aim A A statement which answers the question asked in the experiment Method B What you hope to find out in the experiment Equipment C Information collected during the experiment Conclusion D A drawing of the laboratory items you used for the experiment Results E A set of instructions followed to complete the experiment 10 Use your 30 cm ruler to measure the lengths of the following lines to the nearest mm. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.19 11 Carefully study the drawings of Animal A and Animal B. List three similarities and three differences you can see in these drawings. 12 Steven grew some seeds and, once they had sprouted, he measured their height each day for two weeks and recorded his results in a table. Plot a graph of these results as a line graph on the graph paper below. Day 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Height of plant in cm 1 2 2 3 4 6 11 13 14 16 19 19 19 19 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.20 13 Cassie and Ying are asked to carry out an experiment in which they measure the temperature of water as it is heated. They know that they should write down their method (what they do) and their observations (what they see) very carefully, but they produce the following list by writing on small pieces of paper which then get mixed up in the wrong order. Some of their observations are descriptions of equipment, others are observations of events which happened, and the third type are actions involved in the experiment. a Read the list carefully and write the letter of each observation in its correct category in the table below the list. A The beaker contains 100 mL of water. B The match is struck to light it. C The thermometer initially reads 22oC. D The gas is turned on and let flow for a few seconds to push the air out of the tube. E The Bunsen burner has a base, a barrel or tube, a collar and a long rubber hose. F The gauze and the tripod are very hot. G The collar is adjusted to give the heating flame. H The temperature of the water is measured using a thermometer. I The thermometer is clamped in place so that the bulb is in the centre of the water in the beaker. J 100 mL of water is poured into the beaker from the measuring cylinder. K The Bunsen collar is turned to close the air hole. L The gauze is red above the flame. M Bubbles form in the water and rise to the surface. N The beaker is placed on the tripod above the gauze. O The gauze and tripod are cooled off under water from the laboratory tap. P The Bunsen burner is connected to the gas tap. Q The thermometer finally reads 101oC. R The surface of the water is moving in a rolling motion. S The match is held near the Bunsen and the gas allowed to light. T After 1.5 minutes of heating the thermometer reads 60oC. U The Bunsen is placed under the tripod and the gauze to heat the water. V The Bunsen has a hole in its collar which must be closed before being lit. Descriptions of equipment Observations of events during the experiment Descriptions of actions involved in the experiment b Cassie and Ying have to write their method into their practical report. Using your list of actions involved in the experiment, write down the letters in the correct order for Cassie’s and Ying’s method. c Draw a diagram of the equipment set up for the experiment. Name one piece of equipment needed but not mentioned in the observations. d Water has a boiling point of 100oC but Cassie and Ying find that their water boils at a higher temperature. Can you think of a logical reason for this difference? © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.21 Answers to additional topic test: Recall of knowledge 1 2 3 4 5 6 7 C C B C C 1—D; 2—E; 3—A; 4—B; 5—C. 8 9 Aim: B Method: E Equipment: D Conclusion: A Results: C 10 a 68 mm b 97 mm © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.22 11 Similarities Differences 1 Both have long tails 1 Size of eyes—animal B has larger eyes 2 Both have four legs 2 Animal B has obvious ears 3 Both have eyes 3 Animal A has a rough ridge down its back 12 13 a Descriptions of equipment Observations of events during the experiments Descriptions of actions involved in the experiment A, E, F, L, V M, C, Q, R, T B, D, G, H, I, J, K, N, O, P, S, U b J, N, P, K, B, D, S, H, I, G, U, O © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Topic Test 1.23 c retort stand not mentioned d Many answers are possible—e.g. students made a parallax error reading, the thermometer was not accurate, etc. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.24 PART 3 ADDITIONAL ACTIVITIES AND EXERCISES A1.1 Activity: How well can you observe? Aim To observe then discuss, or draw, some diagrams. Part A: Using notes and drawings Method 1 Take exactly one minute to study the diagram of the house shown here. You can take whatever notes or drawings you like during this time. Have a partner time you. 2 Turn over the page. (Your partner can still look at the diagram, but not let you see it.) 3 Your partner will ask the following questions. You have 10 seconds to answer each one. Score 1 for each correct response, and 0 for an incorrect one. a How many storeys does the house have? b Is there an arch over the front door? c How many chimneys are there on the roof? d How many columns are at the front of the house? e How many steps are there to the front landing? f How many windows are there on the second storey at the front? g There are two differences between the windows on the top floor and the windows on the ground floor. What are they? (1 point for each difference) h Draw a diagram of the roof. i How many panes of glass are there in the front door? © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.25 Results 1 How well did you score? 2 Comment on your powers of observation. Part B: Using notes only Method 1 Take exactly one minute to study the diagram of the frog. You can take whatever notes you wish, but no drawings, during this time. A partner can time you. 2 Turn over the page. 3 Redraw the diagram as best you can from your notes and from memory. 4 Change places with your partner and repeat. Results 1 How well did you reproduce the diagram? 2 How well does your diagram compare with your partner’s? 3 Comment on your powers of observation. (Your artistic skill is not being measured here.) Conclusion Write a conclusion for this experiment. This should be a generalisation about your powers of observation. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.26 A1.2 Exercise: Additional problem solving questions 1 Shown here is a conversion scale which converts temperature from degrees Fahrenheit (oF) (as used in the USA) to degrees Celsius (oC) (as used in Australia and Europe). a Water boils at 100oC. What is this in degrees Fahrenheit? b Water freezes at 32oF. What is this in degrees Celsius? c A person’s body temperature is close to 37oC. Convert this to degrees Fahrenheit. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.27 2 Here are some graphs. Each graph can be improved. What may be done to them to make them better? (You don’t need to do anything to them, just say what could be done.) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.28 3 Drivers are not the only people killed on our roads. This sector graph gives a breakdown of people killed on Australian roads. Answer true or false to these statements. a Most people killed on our roads are drivers. b About two-thirds of the people killed on our roads are car drivers or passengers. c More than one-quarter of the people killed are pedestrians. d This graph shows that there are more motorcycle riders on our roads than pedal cyclists. 4 Brass is a mixture of two metals: copper and zinc. A certain sample of brass consists of 68% copper and 32% zinc. Which sector graph could best represent this? © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.29 A1.3 Literacy exercise: Three level reading exercise—the Bunsen burner Read the following and answer the questions which follow. The Bunsen Burner Nearly every student has seen and used a Bunsen burner to heat substances in a science laboratory. Most students will know that the Bunsen burner was named after its inventor, Robert Bunsen (1811–1899), a German chemist. However, very few students will know that the burner was invented as a tool to help complete a series of experiments which began the modern science of spectroscopy, in which the properties of matter are analysed using light. When it interacts with matter, light is spread out into bands of colour like a rainbow, just like light forming a rainbow in the sky when it passes through raindrops. In 1854, another German physicist Gustav Kirchhoff (1824–1887) was working at the University of Heidelberg with the already well-known Robert Bunsen. Kirchhoff’s experiments were about the ways substances behaved when heated until they gave off light. Bunsen and Kirchhoff would then analyse the light given off by the heated chemicals by passing it through a glass prism and a narrow slit, breaking the light into its spectrum of coloured lines. The key to this experiment was the invention of the burner in 1857. The important features of the Bunsen burner’s flame are: the burner’s flame produces very little light of its own; and it is a very effective means of heating chemicals. These features gave Kirchhoff and Bunsen the ability to study the light from the glowing chemicals without interference from the light of the dim blue burner flame. Using their spectrometer, Kirchhoff and Bunsen found that each chemical heated in the flame gave off a distinct pattern of coloured lines, which became known as a ‘spectral signature’. In today’s scientific world, spectroscopy is used as a standard technique in analytical chemistry to identify unknown substances and even work out the composition of stars and galaxies; while Bunsen burners, with very few modifications from the original design, are still the most basic of laboratory equipment. Answer T (true) or F (false) to the following questions. Level 1: Recall of information 1 2 3 4 The Bunsen burner is named after its inventor, Robert Bunsen. Robert Bunsen was a physicist. Gustav Kirchhoff worked in Germany. Each chemical heated in a flame gives off a distinct pattern of coloured lines when viewed through a spectrometer. 5 Spectroscopy is not used in science today. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.30 Level 2: Comprehension 6 Kirchhoff was 30 years of age when he worked at the University of Heidelberg. 7 A spectrometer contains a glass prism and a narrow slit through which light can pass. 8 Since Bunsen burner flames produce little light of their own, they do not interfere with the ‘spectral signatures’ of chemicals being heated in them. 9 Robert Bunsen was 78 when he died. 10 Robert Bunsen was born 13 years before Kirchhoff. 11 The Bunsen burner was invented to make an experiment easier. Level 3: Applications and generalisations (correlations) 12 Spectroscopy may be the way we discover organic (carbon-based) molecules in space. 13 Many important discoveries hav e had many and varied uses. 14 Previously unknown chemicals cannot be identified by spectroscopy. 15 Substances can be identified as pure and impure by spectroscopy. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.31 A1.4 Literacy exercise: Sources of scientific words Scientific words in English are divided into three groups according to their origins: words taken from ordinary English words taken from another language (usually unchanged) words which have been invented (the largest group) Scientists do not usually invent words ‘out of their head’ but take bits and pieces from Greek, Latin or other languages to build up the word. Below is a table of beginnings and endings as well as their meanings. Beginning/ending Meaning a-, an- not, without, lacking (Gk, a-, an-) alg-, -algia, -algesia pain (Gk algos, algesis) bi-, bio-, -be life (Gk bios) conch-, conchi-, concho- a shell (L. concha ; Gk konche) derm-, dermat-, dermato- the skin (Gk derma, dermat- ) -genesis, genes-, -geny coming into being, origin, formation (Gk genesis) geo-, ge- the Earth (Gk ge) -logy, (-ology) study of limn- , limno- a lake, freshwater (Gk limne) zoo-, zoa-, -zoon, -zoic, -zoid an animal (Gk zoon, a living being, an animal) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.32 Using only the table of scientific word beginnings/endings and meanings on the previous page, give your meaning of the following words. Then, using a dictionary, write down the standard meaning of each word. Word Your meaning Dictionary meaning 1 Dermatology 2 Biogenesis 3 Zoology 4 Limnology 5 Analgesia 6 Geology 7 Abiotic 8 Use the dictionary to find 10 words and their meanings which contain the following endings or beginnings: zoo-, -zoa, -zoon, -zoic, -zoid. 9 The word ‘azote’ (meaning ‘without life’) was originally proposed as the name for nitrogen. Could you suggest a reason for this name? Can you find out which scientist proposed this name? © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.33 A1.5 Puzzle: Spelling/wonderword—investigations Spelling list—find the following words in the grid below. corrosive glassware burner method explosion chemical hottest aim accident toxic tripod gas laboratory analyse gauze tap common contaminate spatula beaker sense pollute burn sink safety infect cylinder funnel goggles destroy hot danger barrel sooty drink solid rubber look waste Bunsen filter C H E M I C A L H I D K C N I N F E C T O B C N E S N U B C N D L O O K G X B H R E H L G L P B U I E E K T M L B U T A R A I M Y O I A S E N S E G I M R H L V O K F I L T E R N K L T S A N N O O E E S E N L A B O R A T O R Y S A T E N T T I R U F U N N E L O S O C A T T D N R A V T A P B L S L G H A Y O E S O E T I N E X P L O S I O N E F J S A H D B A P I D U L C N O C R O T E T W T I G U N O M E T O O U L M U L T T O E C X L R A D A Z E T O X I C B C M Y M C M G U N L F T U K N I R D I B R S P A T U L A E Y I N A C Y L I N D E R D A N G E R E R S F O G L A A S W A R E O G O G G L E S E T C © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.34 A1.6 Literacy and numeracy exercise: Puzzleword The table below contains 36 letters which spell out words from Chapter 1, Investigations. Each letter has a number value found by adding together its row value and column value—e.g. P has a value of 3 + 3 = 6 (third row, third column). But B could be 1 + 2, 1 + 5 or 4 + 6. The same number is not the same letter; and each letter in the table is used once. Work out the correct letters from the possibilities in the table so that you can spell out six words. 1 2 3 4 5 6 1 R B R T B N 2 A K N E I M 3 T R P T U E 4 A S E R A B 5 F N U H O E 6 G L S E D E 6 8 5 7 12 2 3 10 9 4 7 8 6 7 8 5 11 4 7 3 9 4 5 6 10 8 7 6 9 5 8 6 7 9 10 11 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.35 A1.7 Literacy exercise: Crossword puzzle Across clues 2. Includes beakers, cylinders and test tubes 4. Study carefully 5. Very poisonous 6. The steps used to complete an experiment 10. Comes from the gas tap 13. A special spoon used by scientists 14. A chemical which damages the skin is _____ 17. The cylindrical part of the Bunsen burner 21. After the heating experiment, the tripod is _____ 23. If one of these happens, tell the teacher immediately 24. Using the same spatula for two chemicals will _____ the second one Down clues 1. Used when you filter muddy water 2. Should be worn when carrying out an experiment 3. The word describes the yellow Bunsen flame 7. Being careful will minimise the ______ 8. Used to measure liquids 9. Could be for water or gas 10. Placed between the tripod and the beaker 11. Never throw solid wastes into the ____ 12. To observe 15. Mixing unknown chemicals together may make an ______ mixture 16. The name of a science classroom 18. Pouring chemicals into the sink may ____ the water system 19. The yellow flame is also called the _____ flame 20. A virus can _______ many people 22. The problem being solved by an experiment © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.36 A1.8 Literacy exercise: Barrier exercise (oral activity) Students work in pairs. One student has the page with the ‘across’ words filled in, and the other has the page with the ‘down’ words filled in. In turn they give each other clues to enable them to complete the grid. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.37 Down clues Down clues 1. Used when you filter muddy water 2. Should be worn when carrying out an experiment 3. The word describes the yellow Bunsen flame 7. Being careful will minimise the ______ 8. Used to measure liquids 9. Could be for water or gas 10. Placed between the tripod and the beaker 11. Never throw solid wastes into the _____ 12. To observe 15. Mixing unknown chemical together may make an ______ mixture 16. The name of a science classroom 18. Pouring chemicals into the sink may ____ the water system 19. The yellow flame is also called the _____ flame 20. A virus can _______ many people 22. The problem being solved by an experiment © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.38 Across clues Across clues 2. Includes beakers, cylinders and test tubes 4. Study carefully 5. Very poisonous 6. The steps used to complete an experiment 10. Comes from the gas tap 13. A special spoon used by scientists 14. A chemical which damages the skin is ______ 17. The cylindrical part of the Bunsen 21. After the heating experiment, the tripod is _____ 23. If one of these happens, tell the teacher immediately 24. Using the same spatula for two chemicals will _____ the second one © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.39 Answers to additional activities and exercises Answers to A1.2 Exercise: Additional problem solving questions 1 a 212oF b 0oC c 98oF 2 Improvements: (a) Make labels on segments written the same way; add title (b) Add label to the horizontal axis © Give segments different shading; add title (d) Add units to horizontal scale (e) Add scale units to vertical axis; make horizontal scale linear 3 a T b T c F d F 4 graph a Answers to A1.3 Literacy exercise: Three level reading exercise—the Bunsen burner 1 T 2 F 3 T 4 T 5 F 6 T 7 T 8 T 9 F 10 T 11 T 12 T 13 T 14 F 15 T © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.40 Answers to A1.4 Literacy exercise: Sources of scientific words Word Your meaning Dictionary meaning 1 Dermatology Study of skin The study of skin and its diseases 2 Biogenesis The origin of living things *Synthesis of chemical substances by living matter *Hypothesis that living matter arises only from living matter 3 Zoology The study of animals Science of animal structure, physiology, classification, habits and distribution 4 Limnology The study of lakes or fresh water The study of the physical phenomena of lakes and other fresh water 5 Analgesia Without pain Absence or relief of pain 6 Geology The study of the Earth Science of the Earth’s crust, its strata and their relations and changes 7 Abiotic Not living, without life Devoid of life 8 Word Meaning Zoo Zoological gardens Zoolatry Worship of animals Zooplankton Plankton consisting of animals Zooid A cell resembling but not being an animal Zoophyte Plant-like animal especially coral, jellyfish or sponge Protozoa The ‘first animals’—e.g. amoeba, paramecium Cenozoic ‘recent life’—the era from about 60 million years ago to present day Zoolite ‘animal stones’—a fossil animal; a fossilised animal substance Spermatozoan A ‘seed animal’—the typical male sex cell consisting usually of a head (with a nucleus), a thin body and a tail. Zoogeography The study of the distribution of animals on the Earth 9 Lavoisier found that the gas which remained after he had removed the oxygen from air would not support life. He suggested the name ‘azote’. Chaptal renamed the gas ‘nitrogen’. The term ‘azote’ fell into disuse but the azo- unit is still used to indicate nitrogen in a chemical compound (e.g. hydrAZine). Answers to A1.6 Literacy and numeracy exercise: Puzzleword The six words are: burner beaker filter gas tap Bunsen method © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Additional Activities 1.41 Answers to A1.7 Literacy exercise: Crossword puzzle © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.42 PART 4 SELECTED TEXT AND DIAGRAMS—PRINT MASTERS These print masters are selected diagrams and charts from the student book which can be printed out and photocopied for use as worksheets or overhead transparencies. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.43 Figure 1.15 Match the haz-chem symbols to their descriptions and meanings. (Problem solving 1.1) Symbol Description Meaning A H Toxic O Can give off harmful radiation B I Oxidising agent P Can easily burn C J Harmful Q Poisonous D K Flammable R Can help fires get worse or spread E L Radioactive S May explode F M Corrosive T Can affect health if breathed in or absorbed through the skin G N Explosive U Can burn skin and clothes © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.44 The Bunsen burner. © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.45 Figure 1.55 Complete this column graph. (Problem solving 1.4) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.46 Figure 1.56 Complete this sector graph. (Problem solving 1.4) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Print Masters 1.47 Figure 1.57 Complete this bar graph. (Problem solving 1.4) © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 1 Contents for Chapters 2-11 of Science Tracks 7 Teacher Resource CD-ROM CHAPTER 2: CLASSIFICATION OF LIVING THINGS Part 1 Answers to text questions Knowledge and understanding (pages 46–47) Language focus (page 47) Practical activity 2.1: Classification of screws (pages 48–50) Practical activity 2.2: Sorting the class (pages 50–51) Problem solving 2.1 (pages 52–53) Homework set 2.1: Classification (pages 53–55) Knowledge and understanding (page 59) Language focus (pages 59–60) Activity 2.1: Life forms on Kryptos-3 (pages 60–62) Practical activity 2.3: Characteristics of living things (pages 62–64) Problem solving 2.2 (pages 64–65) Homework set 2.2: Living things (pages 66–67) Activity 2.2: Classifying by environment (page 68) Technical literacy exercise (pages 69–70) Computer exercise (page 70) Current issues (page 71) Activity 2.3: Using a wide range of keys (pages 71–72) Topic Test (pages 73–76) 2.3 2.3 2.4 2.5 2.5 2.6 2.7 2.8 2.8 2.9 2.10 2.12 2.13 2.13 2.14 2.15 2.16 2.17 Part 2 Additional topic test Additional topic text: Classification of living things Answers to additional topic text: Classification of living things 2.19 2.23 Part 3 Additional activities and exercises A2.1 Activity: Creating a branching key of Australian beetles Students use diagrams of beetles to create a key A2.2 Activity: Writing a key to identify Australian beetles Students read descriptive passages to create a key A2.3 Literacy activity: Creative writing Students use suncreeen labels to write a pamphlet about the dangers of the sun A2.4 Activity: Cut and paste—locks and keys Students use a classification key to match diagrams of locks and keys 2.25 2.26 2.27 2.28 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 2 A2.5 Activity: Fantasy birds A cut and paste activity where fantasy birds are created. A key is then used to name these birds A2.6 Puzzle: Spelling/wonderword—classification of living things Spelling words are located in the wonderword Answers to additional activities and exercises 2.30 2.31 2.32 Part 4 Selected text and diagrams—print masters Screws to classify (Practical activity 2.1) The Australian fake? (Technical literacy exercise) Diagrams of Australian beetles (A2.1) Sunscreen labels (A2.3) Keys to classify (A2.4) Diagrams of various locks (A2.4) Classification key for keys (A2.4) Fantasy birds (A2.5) Classification key for fantasy birds (A2.5) 2.35 2.36 2.37 2.38 2.39 2.40 2.41 2.42 2.43 CHAPTER 3: PARTICLES OF MATTER Part 1 Answers to text questions Knowledge and understanding (page 82) Language focus (page 82) Practical activity 3.1: Modelling solids, liquids and gases (pages 83–84) Practical activity 3.2: Atoms and molecules (pages 85–86) Problem solving 3.1: Particles of matter (page 87) Homework set 3.1: Particles (page 88) Knowledge and understanding (pages 92–93) Language focus (pages 93–94) Practical activity 3.3: Compressing the states of matter (pages 94–95) Practical activity 3.4: Constructing and using a gas thermometer (pages 96–97) Practical activity 3.5: Diffusion (pages 97–99) Practical activity 3.6: Determining the freezing point of wax (pages 99–100) Problem solving 3.2: Solids, liquids and gases (pages 100–102) Homework set 3.2: Properties of the states of matter (pages 102–103) Knowledge and understanding (pages 104) Technical literacy exercise (page 105) Computer exercise (page 106) Topic test (pages 107–110) 3.3 3.3 3.3 3.4 3.5 3.6 3.7 3.8 3.8 3.8 3.9 3.9 3.10 3.11 3.11 3.12 3.13 3.14 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 3 Part 2 Additional topic test Additional topic test: Particles of matter Answers to additional topic test: Particles of matter 3.16 3.20 Part 3 Additional activities and exercises A3.1 Literacy activity: Current issues—Buckyball ‘transistor’ A comprehension passage based on nanotechnology A3.2 Practical activity: Sublimation Information and experiments involving sublimation A3.3 Exercise: Language focus and homework Additional language focus and homework questions are provided A3.4 Puzzle: Spelling/wonderword—particles of matter Students locate words from the chapter in the wonderword puzzle A3.5 Literacy activity: Definitions help to find a secret word Definitions of key terms help to provide letters for a secret word Answers to additional activities and exercises 3.22 3.23 3.26 3.27 3.28 3.29 Part 4 Selected text and diagrams—print masters Figure 3.14 Particles of common substances. (Problem Solving 3.1) Figure 3.40 Concept map for the states of matter. (Technical literacy exercise) What is matter? (Computer exercise) 3.32 3.33 3.34 CHAPTER 4: FORCES OF NATURE Part 1 Answers to text questions Knowledge and understanding (pages 115–188) Language focus (pages 118–119) Practical activity 4.1: Using a force meter (pages 120–122) Practical activity 4.2: Frictional forces (pages 122–124) Problem solving 4.1: Measuring forces (pages 125–127) Homework set 4.1: Working with forces (pages 127–129) Knowledge and understanding (pages 133–134) Language focus (pages 134–135) Practical activity 4.3: Investigating magnets and magnetic fields (pages 135–138) Problem solving 4.2: Magnetic fields (pages 138–140) Homework set 4.2: Gravity and magnets (pages 140–141) Knowledge and understanding (pages 142–143) Practical activity 4.4: Electromagnets (pages 144–145) 4.2 4.3 4.4 4.5 4.5 4.7 4.10 4.11 4.11 4.13 4.15 4.15 4.16 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 4 Topic test (pages 146–150) 4.17 Part 2 Additional topic test Additional topic test: Forces of nature Answers to additional topic test: Forces of nature 4.20 4.27 Part 3 Additional activities and exercises A4.1 Literacy activity: Current issues—using physics to advantage Comprehension passage concerning the reduction in frictional drag in women’s swimsuits A4.2 Literacy activity: Read and retell—the history of magnetism Group work and class activity to help students focus on key ideas A4.3 Literacy activity: Language exercise—using the history of magnetism Students discover verbs and adjectives by re-reading a text A4.4 Puzzle: Circular word puzzle—forces Students locate key words in this circular puzzle Answers to additional activities and exercises 4.30 4.31 4.33 4.34 4.36 Part 4 Selected text and diagrams—print masters Figure 4.27 Column graph to complete (Problem solving 4.1) Figure 4.31 Action and reaction forces (Homework set 4.1) Figure 4.52 Gravitational effects of the Sun and Moon on tides (Problem solving 4.2) Diagram of a driver and the forces which act on him to bring him to rest during a collision 4.38 4.39 4.40 4.41 CHAPTER 5: PATTERNS IN THE SKY Part 1 Answers to text questions Knowledge and understanding (pages 158–159) Language focus (pages 159–160) Practical activity 5.1: Sun in the sky (pages 160–163) Practical activity 5.2: Plotting star positions on star charts (pages 163–166) Problem solving 5.1: Locating the stars (pages 166–169) Homework set 5.1: Investigating the stars (page 169) Knowledge and understanding (pages 175–176) Language focus (pages 177–178) Practical activity 5.3: Modelling the Solar System (pages 178–180) 5.3 5.5 5.5 5.5 5.7 5.8 5.9 5.10 5.11 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Practical activity 5.4: Modelling retrograde motion in a square solar system (pages 181–182) Problem solving 5.2: Orbits and revolutions (pages 183–184) Homework set 5.2: Planets and other bodies (pages 184–185) Practical activity 5.5: Moon phases (pages 186–188) Technical literacy (pages 188–190) Topic test (pages 191–193) Contents page 5 5.11 5.11 5.12 5.13 5.13 5.14 Part 2 Additional topic test Additional topic test: Under southern skies Answers to additional topic test: Under southern skies 5.15 5.19 Part 3 Additional activities and exercises A5.1 Exercise: Additional questions relating to text content More questions based on history of astronomy, the zodiac and constellations A5.2 Literacy activity: Mars and science fiction Comprehension exercise on the relationship between H.G. Wells’ The War of the Worlds and attitudes towards Mars at the time A5.3 Activity: Luna—the Moon Information text on the Moon, including Apollo space missions, myths and legends, and how the Moon orbits the Earth A5.4 Internet Activity: Sourcing current information on astronomy Web sites for locating current data on space missions A5.5 Activity: Current issues—Galileo–Europa mission Comprehension exercise on the Galileo probe to Europa A5.6 Puzzle: Puzzle words—the southern sky Answers in the puzzle help reveal a message about the Southern sky A5.7 Language activity: The phases of Venus Comprehension and cloze passages about Venus and its phases Answers to additional activities and exercises 5.22 5.23 5.25 5.28 5.29 5.30 5.31 5.34 Part 4 Selected text and diagrams—print masters Figure 5.5 Celestial sphere and ecliptic Figure 5.15 Path of the Sun at different times of the year (Knowledge and understanding, pages 158–159) Figure 5.26 Sky diagram (Practical activity 5.2) Figure 5.30 Positions of the stars of the Southern Cross in space (Problem solving 5.1) Figure 5.46 Square solar system (Practical Activity 5.4) 5.39 5.40 5.41 5.42 5.43 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 6 CHAPTER 6: STUDENT RESEARCH PROJECT 1 Part 1 Answers to text questions Knowledge and understanding (pages 196–197) Language focus (pages 197–198) Problem solving 6.1: Thinking (pages 198–199) Homework set 6.1: Observations, inferences, and generalisations (page 200) Knowledge and understanding (page 201) Homework set 6.2: Writing reports (page 206) Topic test (pages 207–208) 6.2 6.3 6.4 6.4 6.5 6.5 6.6 Part 2 Additional topic test Additional topic test: Student research project Answers to additional topic test: Student research project 6.8 6.10 Part 3 Additional activities and exercises A6.1 Activity: What is a ‘typical’ scientist? Class discussion about what a scientist is A6.2 Puzzle: Crossword Crossword concerning the branches of science A6.3 Puzzle: Wheelwords Key words in this topic are found in each wheel Answers to additional activities and exercises 6.11 6.12 6.13 6.14 Part 4 Selected text and diagrams—print masters Figure 6.5 Steps of a scientific enquiry (Knowledge and understanding, pages 196–197) Figure 6.8 An experiment report 6.16 6.17 CHAPTER 7: LIFE FORMS Part 1 Answers to text questions Knowledge and understanding (page 214) Language focus (page 214) Practical activity 7.1: Diversity of life forms (page 215) Practical activity 7.2: Observing living things at home (pages 216–217) Problem solving 7.1: Characteristics of living things (pages 217–218) 7.2 7.2 7.2 7.3 7.3 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Homework set 7.1: Classifying living things (pages 218–219) Knowledge and understanding (page 224) Language focus (pages 224–225) Practical activity 7.3: Classifying vertebrates (pages 225–227) Practical activity 7.4: Pregnancy in mammals (pages 227–228) Problem solving 7.2: More about vertebrates (pages 228–229) Homework set 7.2: Vertebrates and their features (pages 231–232) Knowledge and understanding (page 234) Technical literacy exercise (pages 234–235) Topic test (pages 238–240) Contents page 7 7.4 7.4 7.6 7.6 7.7 7.9 7.10 7.11 7.12 7.12 Part 2 Additional topic test Additional topic test: Life forms Answers to additional topic test: Life forms 7.14 7.18 Part 3 Additional activities and exercises A7.1 Literacy activity: Cloze activity—fish (Agnatha and Placoderms) Cloze passage concerning lesser known fish A7.2 Literacy activity: Current issues—microbes cause disease of frogs Comprehension passage about the world wide decline in frog populations A7.3 Puzzle: Spelling/wonderword—life forms A wonderword using words from the topic A7.4 Puzzle: Piewords A difficult puzzle where students locate letters from pie segments to create key words Answers to additional activities and exercises 7.19 7.20 7.22 7.23 7.24 Part 4 Selected text and diagrams—print masters Table: The features of vertebrates (Problem solving 7.2) Figure 7.27 Vertebrate classification key (Problem solving 7.2) Sam’s adventures in the five kingdoms (Computer exercise) 7.27 7.28 7.29 CHAPTER 8: MIXTURES AND THEIR SEPARATION Part 1 Answers to text questions Knowledge and understanding (pages 246–247) 8.3 Language focus (pages 247–248) 8.4 Practical activity 8.1: Homogeneous and heterogeneous mixtures (pages 248–250) 8.4 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 8 Practical activity 8.2: Testing different solvents (pages 250–251) Problem solving 8.1 (pages 252–253) Homework set 8.1 (page 253) Knowledge and understanding (pages 258–259) Language focus (pages 259–260) Practical activity 8.3: Filtration (pages 260–262) Practical activity 8.4: Evaporation and crystallisation (pages 262–263) Practical activity 8.5: Distillation (pages 263–264) Problem solving 8.2: Separating substances (pages 265–266) Homework set 8.2: More separation (pages 268–270) Knowledge and understanding (pages 271–273) Technical literacy exercise (page 274) Topic test pages (277–279) 8.5 8.6 8.7 8.8 8.10 8.11 8.11 8.12 8.12 8.14 8.16 8.17 8.17 Part 2 Additional topic test Additional topic test: Mixtures and their separation Answers to additional topic test: Mixtures and their separation 8.19 8.23 Part 3 Additional activities and exercises A8.1 Practical activity: A salty problem Student teams design and carry out an experiment to extract salt from rock salt A8.2 Practical activity: Problems of separation Students use techniques of separating mixtures to solve three problems A8.3 Literacy activity: Comprehension—colloids Comprehension passage about colloids and their properties A8.4 Practical activity: Colloids Preparing colloidal suspensions and investigating their properties A8.5 Literacy activity: Current issues—technology solves the problem of the bitter orange New technology helps to solve a problem in bitter oranges A8.6 Puzzle: Spelling/wonderword—mixtures and their separation A wonderword puzzle which reinforces key words Answers to additional activities and exercises 8.25 8.26 8.27 8.30 8.32 8.34 8.35 Part 4 Selected text and diagrams—print masters Figure 8.35 Particle diagram of filtration (Homework set 8.2) Figure 8.37 Distillation apparatus (Homework set 8.2) Natural resources: Extraction of sulfur (Technical literacy exercise) Figure 8.43 The process of extracting sulfur (Technical literacy exercise) 8.37 8.38 8.39 8.40 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 9 CHAPTER 9: ENERGY IN OUR LIVES Part 1 Answers to text questions Knowledge and understanding (pages 284–285) Language focus (page 285) Practical activity 9.1: Oscillations (pages 286–287) Practical activity 9.2: Heat and light (pages 287–289) Practical activity 9.3: Electrical, sound and chemical energy (pages 289–291) Problem solving 9.1: Mechanical energy and energy sources (pages 292–294) Homework set 9.1: Fossil fuels, types of energy and energy sources (pages 294–295) Knowledge and understanding (pages 298–300) Language focus (pages 300–301) Practical activity 9.4: Energy transformations 1 (pages 301–303) Practical activity 9.5: Energy transformations 2 (pages 304–306) Problem solving 9.2: Energy transformations and hot rocks (pages 306–309) Homework set 9.2: Producing and supplying energy (pages 309–310) Technical literacy exercise (pages 310–312) Computer exercise (pages 312–314) Topic test (pages 315–319) 9.3 9.4 9.4 9.5 9.5 9.6 9.8 9.9 9.10 9.11 9.11 9.12 9.13 9.14 9.17 9.17 Part 2 Additional Topic test Additional topic test: Energy in our lives Answers to additional topic test: Energy in our lives 9.20 9.24 Part 3 Additional activities and exercises A9.1 Activity: Current issues—the girth of a nation A newspaper article is used as a focus for debate A9.2 Activity: Saving energy in the home Comprehension and numeracy exercise on ways of saving energy A9.3 Literacy activity: Language focus—use of past tense Writing sentences using an energy context Answers to additional activities and exercises 9.26 9.28 9.31 9.33 Part 4 Selected text and diagrams—print masters Figure 9.13 Australia’s energy source use (Problem solving 9.1) Figure 9.14 Australia’s energy source reserves (Problem solving 9.1) Report: Historical aspects of energy usage (Technical literacy exercise) 9.37 9.38 9.39 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 10 Table: Common foods (Computer exercise) 9.40 CHAPTER 10: THIS LAND AUSTRALIA Part 1 Answers to text questions Knowledge and understanding (pages 325–326) Language focus (page 326) Practical activity 10.1: Rocks of an ancient land (pages 327–328) Problem solving 10.1: Regions of Australia (pages 329–331) Homework set 10.1: Vegetation (pages 331–332) Knowledge and understanding (page 338) Language focus (page 339) Practical activity 10.2: Modelling a coastal river and delta (pages 340–341) Problem solving 10.2: Moving earth (pages 341–342) Homework set 10.2: Physical and chemical weathering (page 342) Knowledge and understanding (page 344) Language focus (pages 344–345) Practical activity 10.3: Field trip to a sand dune (pages 345–347) Problem solving 10.3: Formation of sand dunes (pages 347–348) Homework set 10.3: About sand dunes (pages 348–349) Current issues (pages 349–351) Options: Science and myth—the Great Barrier Reef (pages 351–352) Technical literacy exercise (page 352) Topic test (pages 353–356) 10.3 10.3 10.4 10.4 10.5 10.6 10.7 10.7 10.8 10.9 10.9 10.10 10.10 10.10 10.11 10.12 10.12 10.13 10.13 Part 2 Additional topic test Additional topic test: This land Australia Answers to additional topic test: This land Australia 10.15 10.17 Part 3 Additional activities and exercises A10.1 Activity: Mapping the beach Interpreting a diagram about organisms in a beach habitat A10.2 Activity: Mangroves and estuaries Use of a scale diagram and comprehension questions about organisms in a mangrove community A10.3 Puzzle: Spelling/wonderword—this land Australia Wonderword to reinforce spelling of key words Answers to additional activities and exercises 10.18 10.20 10.22 10.23 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia Science Tracks 7 Teacher Resource CD-ROM Contents page 11 Part 4 Selected text and diagrams—print masters Figure 10.5 Map of surface rock zones (Practical activity 10.1) Figure 10.7 Rainfall map of Australia (Problem solving 10.1) Figure 10.28 Sand dunes on Stradbroke Island, Queensland (Problem solving 10.3) Figure 10.30 Reef profile (Options: science and myth—the Great Barrier Reef) 10.25 10.26 10.27 10.28 CHAPTER 11: SCIENCE JOURNAL: ISSUES IN SCIENCE Part 1 Answers to text questions Knowledge and understanding (page 360) Language focus (page 361) Problem solving 11.1: The moon (pages 361–362) Problem solving 11.2: Endangered (pages 362–364) Problem solving 11.3: Endangered and extinct birds (pages 364–365) Problem solving 11.4: Coffee and caffeine (pages 367–368) Activity 11.5: Puzzles (page 370) 11.2 11.2 11.2 11.2 11.3 11.4 11.5 Part 2 Additional activities and exercises A11.1 Activity: Jobs or insects? Passage used as a stimulus for class discussion about environmental protection versus jobs A11.2 Activity: The future Designing a poster about our future life in a technological world 11.6 11.7 Part 3 Selected text and diagrams—print masters Figure 11.7 Flowchart of carbon dioxide decaffeination (Problem solving 11.4) 11.9 Figure 11.8 Quantities of caffeine present in various drinks (Problem solving 11.4) 11.10 Figure 11.13 Graph of windspeed versus angle of deflection (Practical activity 11.1) 11.11 © L Thickett, G Thickett, J Stamell 1999. Published by Macmillan Education Australia